Supplemental Digital Content is available in the text
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is characterized by a strong fibrotic stromal reaction and diffuse growth pattern. Peritumoral fibrosis is often evident during surgery but only distinguishable from tumor by microscopic examination. The aim of this study was to investigate the role of clearance of fibrotic stromal reaction at the mesopancreatic resection margin as a criterion for radical resection and preoperative assessment of resectability.
Mesopancreatic stromal clearance status (S-status) was defined as the presence or absence (S+/S0) of fibrotic stromal reaction at the mesopancreatic resection margin. Detailed retrospective clinicopathologic re-evaluation of margin status and preoperative cross-sectional imaging was performed in a cohort of 91 patients operated for pancreatic head PDAC from 2001 to 2011.
Conventional margin positive resection (R+, tumor cells directly at the margin) was found in 36%. However, S-status further divided the margin negative (R0) group into patients with median survival of 14 months versus 31 months (S+ versus S0, P = 0.005). Overall rate of S+ was 53%. S-status and lymph node ratio constituted the only independent predictors of survival. Stranding of the superior mesenteric artery fat sheath was the only independent radiologic predictor of S+ resection, and achieved a 71% correct prediction of S-status.
Mesopancreatic stromal clearance is a major determinant of curative resection in PDAC, and preoperative prediction by cross-sectional imaging is possible, setting the basis for a new definition of borderline resectability.
INTRODUCTION
Only about 20% of patients with pancreatic ductal adenocarcinoma (PDAC) present with localized disease amenable to surgical resection.1 PDAC is characterized by fast local progression and early distant metastasis resulting in one of the worst survival rates among all human cancers.2,3 Even successful surgical resection yields 5-year survival rates of only around 20%.4,5 Moreover, patients continue to succumb to the disease even after prolonged survival (>5 years).6 Virtually all patients experience local and/or metastatic recurrence.7–9
The mere anatomical location of pancreatic head cancer makes conventional radical resection of the local disease, obtaining wide security margins, nearly impossible. Only a narrow space posterior to the pancreatic head and neck separates the pancreas from the adjacent major arterial vessels. This space has been coined “mesopancreas,”10,11 although no clear connective tissue sheaths exist to fully justify the term “meso.”11–13 The mesopancreas contains variable amounts of fatty tissue, parts of the autonomous visceral nervous system, lymphatic vessels, and locoregional lymph nodes. It has been shown10–13 that most margin positive pancreatoduodenectomies are caused by margin positivity in the mesopancreatic area.14–17
While en bloc resection of the superior mesenteric or portal vein (PVR) for tumor adhesion has become standard in many centers,18–21 the superior mesenteric artery (SMA), hepatic artery (HA), inferior caval vein (ICV), and aorta are usually not considered resectable.18,22 Of note, preoperative prediction of resectability by radiologic criteria is sensitive and specific for portal venous, but only to a lesser degree for arterial involvement, where surgical exploration is usually needed in equivocal cases.23,24
During resection, the surgeon is often confronted with extensive peritumoral fibrotic stromal reaction in the mesopancreatic region.17 This results in adhesion to the aforementioned blood vessels and intraoperatively suggests borderline resectability of this tumor. In this situation, sharp dissection right through the fibrotic stroma is often necessary to mobilize the tumor and pancreatic head. Histopathologically, PDAC is characterized by an abundant fibrotic stromal reaction.1,25,26 The role of this peritumoral stroma is currently debated: experimental evidence points toward a supporting role of this stroma in the process of tumor invasion, metastasis, and treatment resistance, however contradictory results have also been published.27,28
Within this context, we strove to investigate the clinical impact of surgical mesopancreatic stromal clearance during resection of pancreatic head cancer. Furthermore, the aim was to predict the achievement of stromal clearance preoperatively by radiologic parameters.
METHODS
Patients and Tissue
Patients operated for pancreatic head PDAC from 2001 to 2011 at the Clinic for General and Visceral Surgery, University Medical Center Freiburg, with histopathological workup at the Institute of Pathology, University Medical Center Freiburg were identified. Permission was obtained from the institutional ethics committee of the University of Freiburg (ref 13/11). All histological samples and corresponding pathological reports were reevaluated independently by 3 experienced pathologists for correctness of diagnosis and resection margin status. Two experienced surgeons reviewed operation reports, clinical and follow-up data. Cases with perioperative death or insufficient material for detailed reevaluation were excluded.
Standard Specimen Workup
A standardized workup for gross sectioning was performed for pancreatoduodenectomy. The biliary, oral and aboral enteric, pancreatic parenchymal, and mesopancreatic resection margins were marked by the surgeon. Extra tissue samples were evaluated on clinical demand. All specimens were transferred to the Institute of Pathology for frozen sectioning and examined by experienced pathologists. Macroscopic tumor masses were measured, tumor localization, infiltration of surrounding structures, and distance to resection margins were documented. The mesopancreatic margin was inked before gross sectioning for histologic orientation. The standard gross sectioning protocol comprised samples of the oral and aboral enteral, biliary, pancreatic circumferential, and parenchymal resection margins, as well as tumor samples in relation to the closest mesopancreatic margin, distal bile duct, main pancreatic duct, Ampulla of Vater, duodenum, and at least 12 locoregional lymph nodes. Tissue samples from the mesopancreatic margin and tumor samples were sliced orthogonal to the resection plane, and parallel to the resection plane from the parenchymal and proximal biliary margin. Resection margins of the splenic artery and vein and 1 sample of the spleen were taken in case of a total pancreatosplenectomy. In case of en bloc superior mesenteric or portal vein resection, tissue specimens of the vessel margins and tumor in relation to the vessel were embedded.
Tissue specimens were formalin fixed and paraffin embedded (FFPE) and 3-μm FFPE tissue slices were haematoxylin and eosin (H&E) stained according to a routine protocol. In case of detection of suspicious cells at the resection margins, immunohistochemistry for Pan-Cytokeratin was performed. Lymph nodes were evaluated separately. The histopathological report included tumor size, grade and WHO type,29 UICC staging (pTNM),30 and microscopic status of all evaluated resection margins. Furthermore, microscopic lymphangiosis (L), hemangiosis (V), and perineural invasion (Pn) were documented.
Conventional Histopathologic Revalidation
All macroscopic and microscopic reports were reviewed independently by 3 experienced pathologists. Reexamination of the H&E stained tissue slides from the tumor and resection margins was performed at 200 and 400-fold magnification. Each resection margin was considered separately. Conventional resection margin status (R-status) was considered positive (R+) when tumor cells were found directly at any margin (zero tumor cell distance rule, see Figure 1).
FIGURE 1.
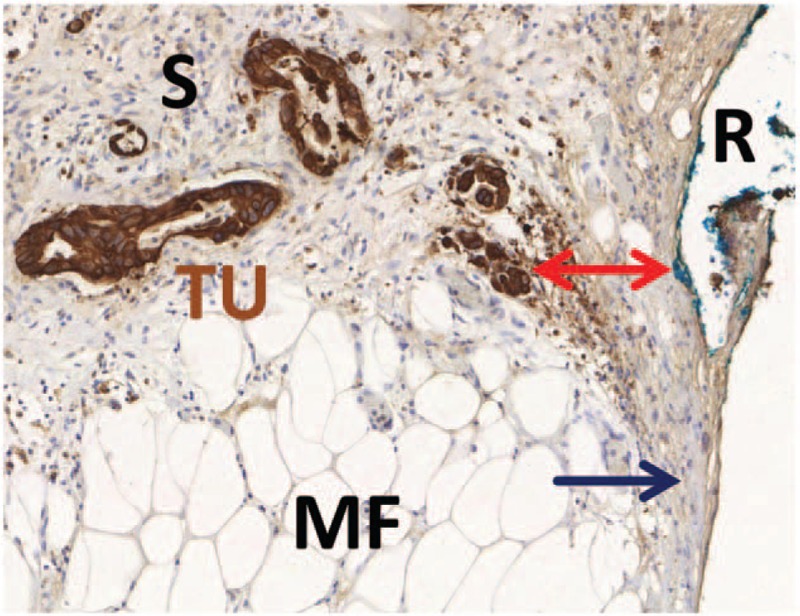
Conventional and histopathological margin status assessment. Example of a tissue slide from the mesopancreatic margin with brown immunohistochemical staining for Pan-Cytokeratin for better visualization of tumor cells. The tumor cells (TU) are surrounded by a dense fibrotic stroma (S) and invade the mesopancreatic fatty tissue (MF). The closest distance to the inked resection margin (R) is marked by a red arrow. Although no tumor cells are found directly at the resection margin, there is broad contact of the fibrotic stroma to the resection margin. Margin status in this case is negative by conventional R-status (R0, zero tumor cell distance rule), but positive by circumferential margin concept (CRM+, 1-mm tumor cell distance rule) and positive by stromal clearance concept (S+, zero stroma distance rule). For details see manuscript text.
Reassessment of Mesopancreatic Resection Margin Status
H&E stained tissue slides of the mesopancreatic margins including samples from portal vein resections were reevaluated in blinded fashion by 2 experienced pathologists and 1 trained surgeon. The mesopancreatic stromal clearance status (S-status) was considered positive (S+) when fibrotic tissue containing fibroblasts and variable amounts of inflammatory infiltrate was identified directly at the inked margin (zero stroma distance rule, Figure 1). Inter-rater agreement for S-status was measured by assessment from 2 independent observers. Margin status according to the circumferential margin (CRM) concept, as originally derived from rectal cancer and adopted to pancreatic cancer,17 was considered positive (CRM+) when tumor cells were found within 1 mm of the inked margin (1-mm tumor cell distance rule, Figure 1).
Assessment of Cross-Sectional Imaging for Prediction of Margin Status
For preoperative staging and assessment of tumor resectability, patients routinely underwent a multiphasic multidetector computed tomography (MDCT) or a gadolinium-enhanced magnetic resonance imaging (MRI) including multiplanar sequences. Preoperative cross-sectional examinations were retrospectively reanalyzed by consensus reading by a clinically experienced radiologist and an experienced surgeon blinded for resection status. The raters evaluated each scan for 6 parameters. Thickness of the fatty tissue sheath between pancreas and superior mesenteric artery (SMA) and inferior caval vein (ICV) as well as distance between tumor and SMA / ICV was measured in millimeters (Figure 2). Furthermore, presence or absence of stranding within the fatty tissue sheaths was documented as positive or negative (Figure 2), and defined as positive when there was no fat sheath of 1 mm or more.
FIGURE 2.
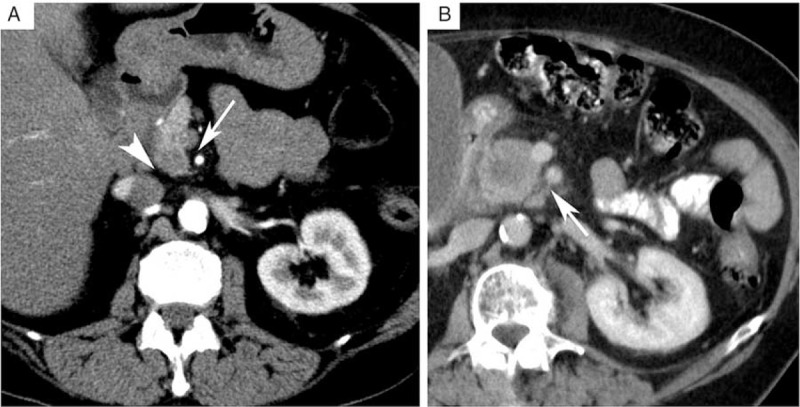
Assessment of radiologic parameters. A, Preoperative contrast enhanced multiphasic multidetector computed tomography (MDCT) demonstrating a normal fatty tissue sheath separating the superior mesenteric artery (SMA, arrow) and the inferior caval vein (ICV, arrowhead) from the pancreas. A small hypovascular/hypodense tumor in the pancreatic head can be seen. B, Preoperative contrast enhanced MDCT demonstrating the presence of stranding, that is increased attenuation, in the SMA fatty tissue sheath (arrow).
Statistical Analyses
Data acquisition and statistics were carried out with MedCalc Statistical Software version 14 (MedCalc Software bvba, Ostend, Belgium). Scale parameters were expressed as median and range, ordinal and nominal variables as absolute numbers, and percent and survival data as estimates by Kaplan–Meier method. Statistical testing was performed with a 2-sided significance level of P = 0.05 by Kappa test for inter-rater agreement, Spearman rank test for correlation, Logrank test, and Cox proportional hazards regression for survival. Binary logistic regression and cross tabulation with calculation of sensitivity, specificity, and predictive values were used for prediction of S-status by radiologic parameters.
RESULTS
Patient Demographics and Operations
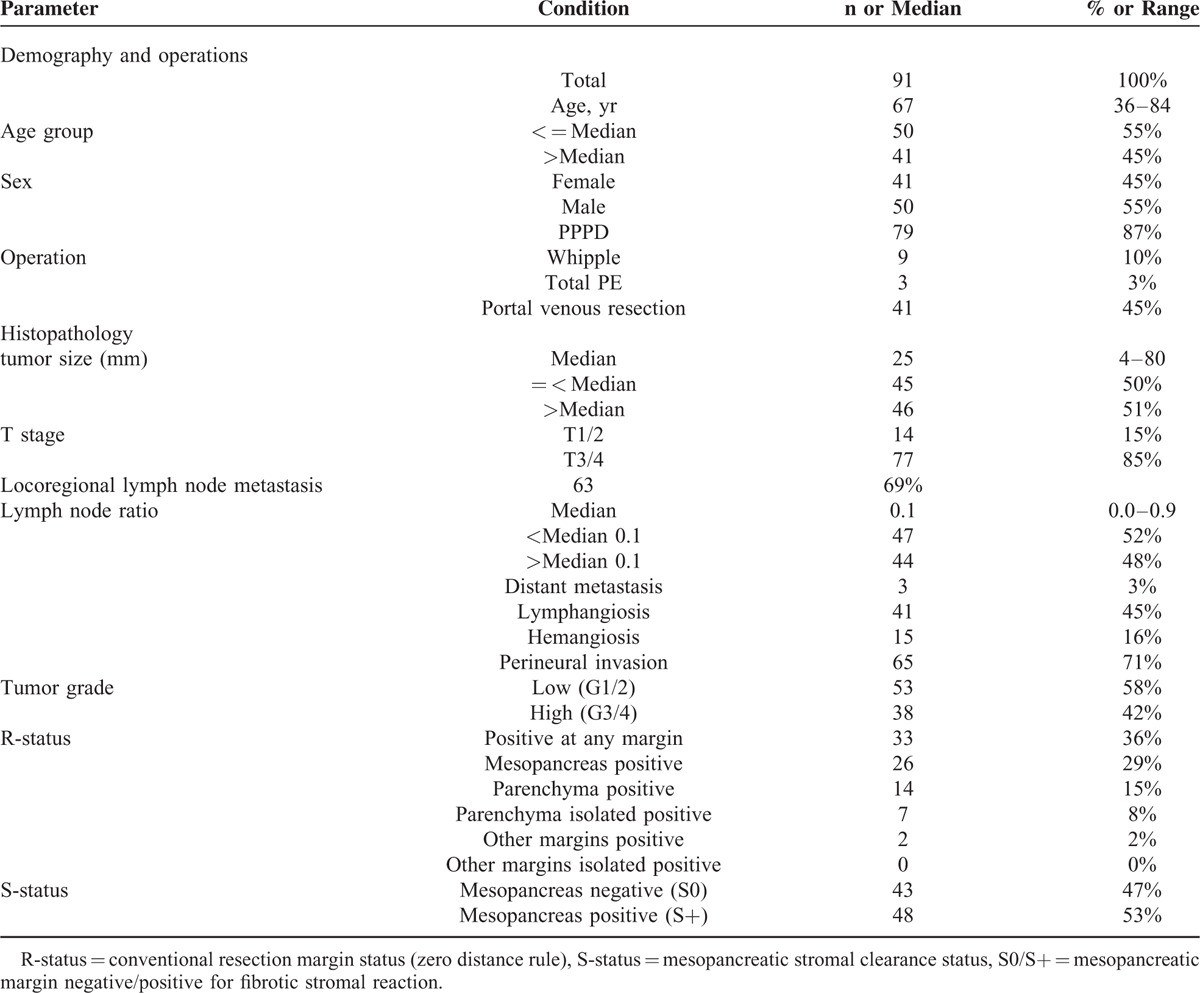
After clinico-pathologic reevaluation, in total n = 91 patients (41 women, 50 men) operated at a median age of 67 years (range, 36–84 years) from 2001 through 2011 for pancreatic head PDAC with sufficient tissue left for reevaluation of resection margins were included (Table 1). Most operations were pylorus-preserving pancreatoduodenectomies (87%, n = 79), and a minority were classic Whipple procedures (n = 9, 10%) and total pancreatectomies (3%, n = 3). En bloc PVR was performed in almost half of the cases (45%, n = 41).
TABLE 1.
Baseline Demography, Operations and Histopathology
Standard Histopathology
All cases were histologically confirmed as PDAC, with about half high-grade (G3/G4) tumors (Table 1). Median tumor size was 25 mm (range, 4–80 mm) and 85% (n = 77) were of T stage 3 or 4. The median number of examined lymph nodes was 14 (range, 2–43). Loco-regional lymph node metastases were detected in 69% (n = 63), with a median lymph node ratio (LNR) of 0.1 (range, 0.0–0.9). Perineural invasion (71%, n = 65) and lymphangiosis (45%, n = 41) were frequent findings, while microscopic hemangiosis (16%, n = 15) was uncommon and distant metastasis (3%, n = 3) was rare.
Histological Margin Status
Conventionally positive margins (R+) were detected in 36 % (n = 33) of resection specimens (Table 1). Most of these were due to a positive mesopancreatic margin (79 %, n = 26). Pancreatic parenchymal margins were positive in 15% (n = 14), and other margins in only 2% (n = 2). Margin positivity did almost always involve a positive mesopancreatic margin, except for 7 cases (8% of all patients) in whom solely the pancreatic parenchymal margin was positive in definitive microscopic workup. In contrast to the conventional R-status, histological reassessment detected fibrotic stromal reaction at the mesopancreatic margins (S+) in more than half of patients (53%, n = 48). Inter-rater agreement for S-status was substantial (kappa value of 0.887, 95% confidence interval 0.791–0.983).
Univariate Survival Analysis
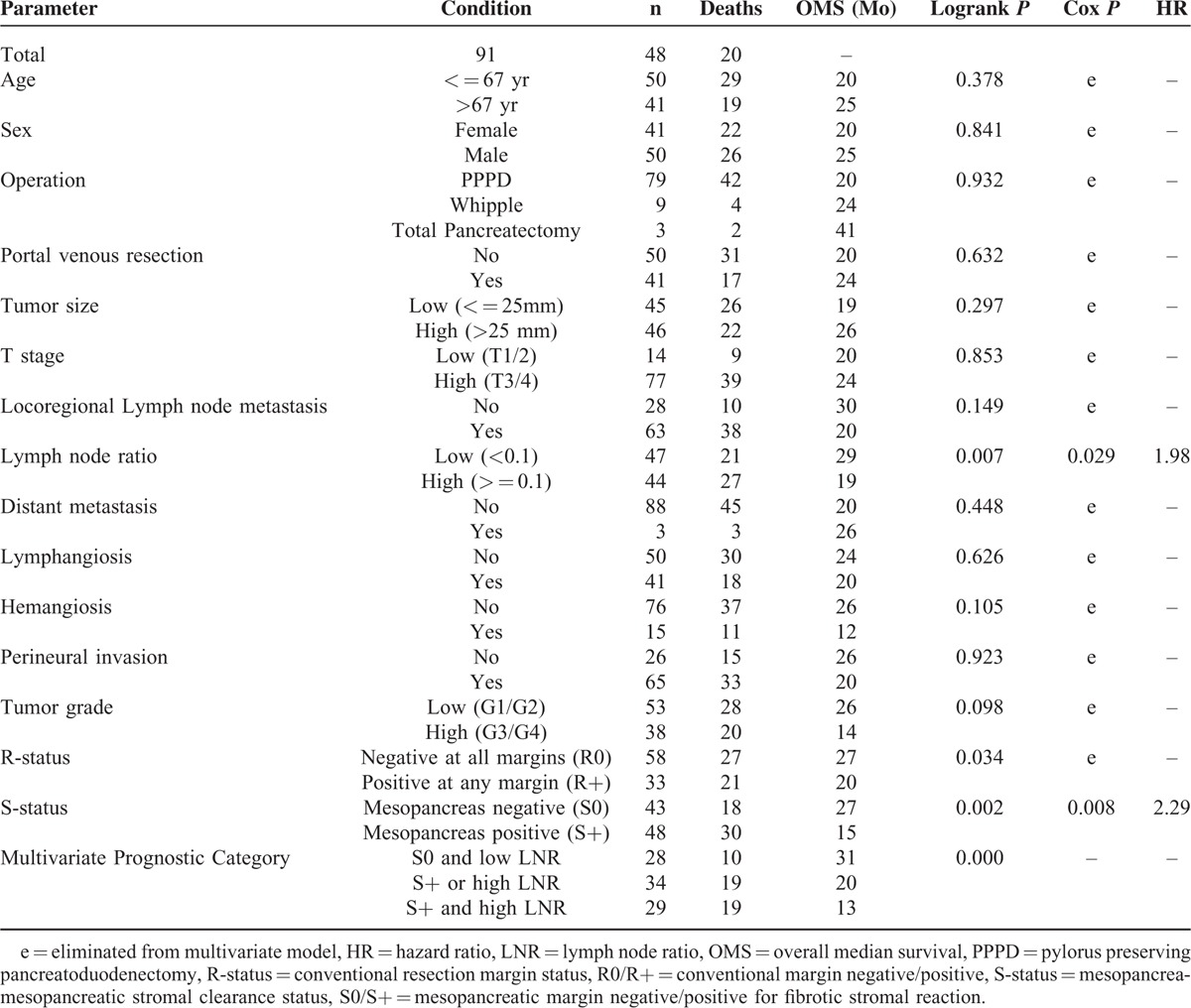
Overall survival was 20 months with 48 deaths during follow-up of 91 patients (median follow-up 13 months). Among all clinico-pathologic parameters, only LNR, R-status, and S-status qualified as predictors of survival (Table 2). Lymph node ratio (cutoff 0.10) distinguished between patients with a median survival of 19 versus 29 months (P = 0.007, Figure 1). Current German guidelines recommend examination of at least 10 lymph nodes.31 However, results were unchanged when patients with less than 10 examined lymph nodes (n = 10) were excluded. The difference in survival was the longest with regard to S-status (positive vs negative, 15 vs 27 months, P = 0.002, Figure 2), while conventional R-status resulted in a considerably smaller difference of 20 versus 27 months (P = 0.03, Figure 2).
TABLE 2.
Univariate and Multivariate Survival Analysis
Multivariate Survival Analysis
In a multivariate proportional hazards model, forward and backward elimination identified only LNR and mesopancreatic S-status as independent predictors of survival after resection of pancreatic head PDAC. Exclusion of 10 cases with less than 10 lymph nodes evaluated did not alter this result. To substantiate these results, survival was compared according to combined assessment of LNR and S-status (Table 2 and Figure 2). In patients with stromal clearance (S0) and low LNR, median survival reached 31 months, and dropped sharply when only 1 parameter (20 months) or both (13 months) were unfavorable (ie, S+ or high LNR, P < 0.001).
Stromal Clearance Status in Conventionally Margin Negative Resections
To further evaluate the prognostic role of the S-status, a subgroup analysis in patients with conventional margin negative resection (R0) was performed (Table 3 and Figure 3). Similarly to the results outlined above, the S-status discriminated sharply between patients with favorable and poor survival (S+ vs S0, median survival 14 vs 31 months, P = 0.005) in this smaller (n = 58) subgroup with a median overall survival of 27 months. Locoregional lymph node metastasis (N0/N+) further subdivided the prognostic categories: Survival after S0 resection with positive locoregional lymph nodes was 29 months, while median survival was not even reached during follow-up in the small group of patients with S0 resection and negative lymph nodes (n = 14, P = 0.01) (Table 3 and Figure 3).
TABLE 3.
Survival Analysis in the R0 Subgroup
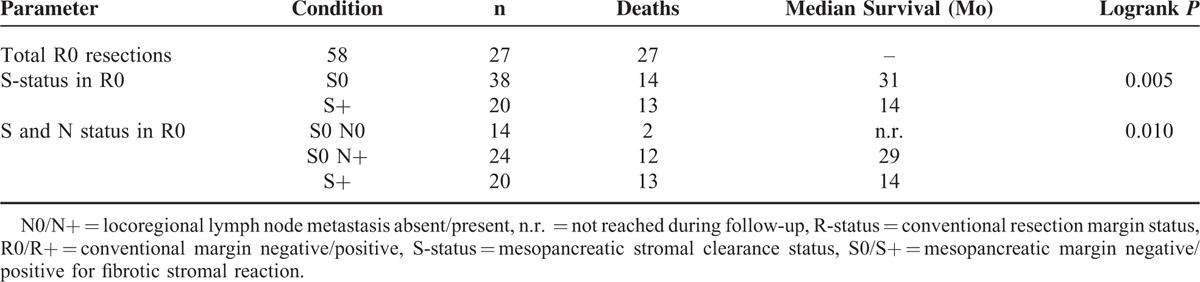
FIGURE 3.
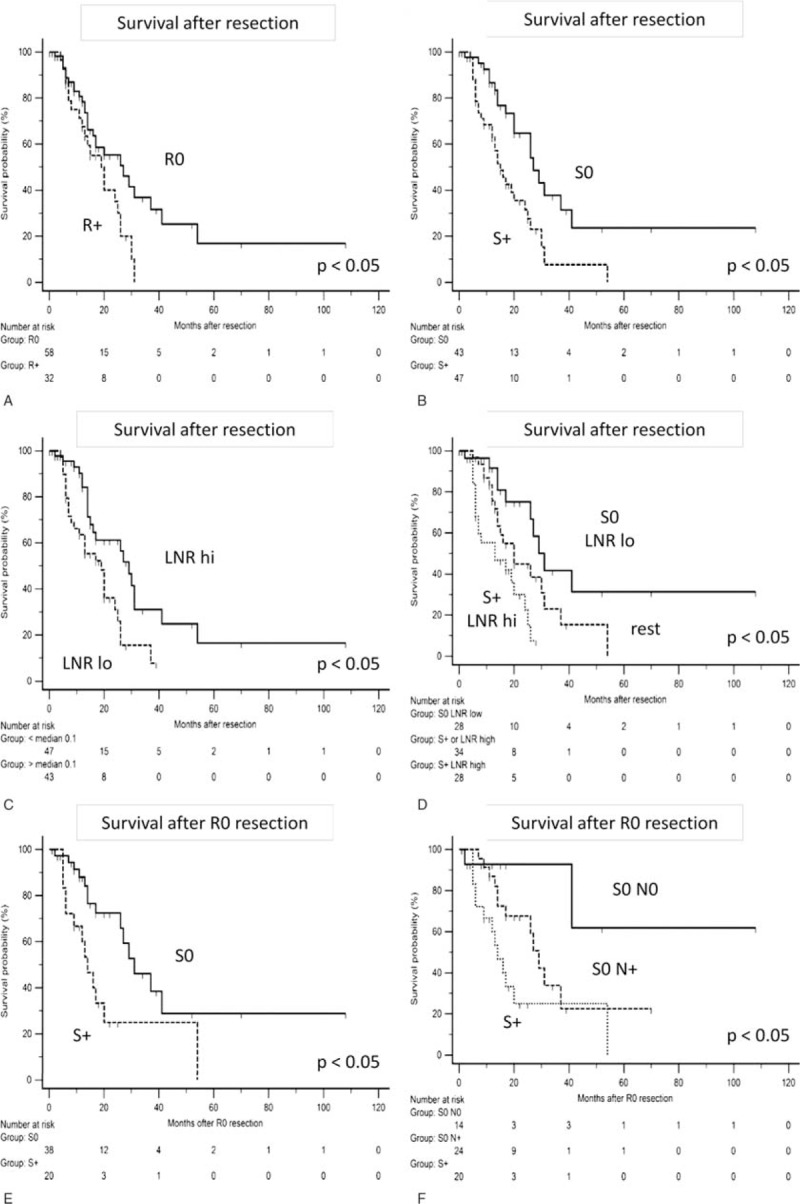
Survival analysis. A–D, Kaplan–Meier plots for comparison of survival after resection of pancreatic head cancer for (A) conventional R0 versus R+ resection, (B) mesopancreatic stroma negative (S0) versus stroma positive (S+) resection, (C) high versus low lymph node ratio (LNR), and (D) patients with S0 margins and low LNR versus patients with S+ margins and high lymph node ratio versus the rest. See also Table 2 for details. E, F, Kaplan–Meier plots for comparison of survival after R0 resection of pancreatic head cancer for (E) mesopancreatic stroma negative (S0) versus stroma positive (S+) resection and (F) patients with S0 resection and no lymph node metastasis (N0) versus S0 resection with lymph node metastasis versus S+ resection. See also Table 3 for details. LNR = lymph node ratio; N0/N+ = locoregional node metastasis absent/present; R-status = conventional resection margin status; R0/R+ = conventional margin negative/positive; S-status = mesopancreatic stromal clearance status; S0/S+ = mesopancreatic margin negative/positive for fibrotic stromal reaction, P values given for 2-sided Logrank test.
Correlation of Mesopancreatic Stromal Clearance With Tumor Biology
To assess the biologic role of the S-status, correlation analysis between S-status and other histopathological parameters (supplemental Table S1) was carried out. Only conventional R-status and LNR showed significant positive correlation with S+ resection. There was no apparent correlation with tumor size or markers of aggressive disease like lymphangiosis, hemangiosis, or perineural invasion.
Correlation of Stromal Clearance Status With CRM Concept
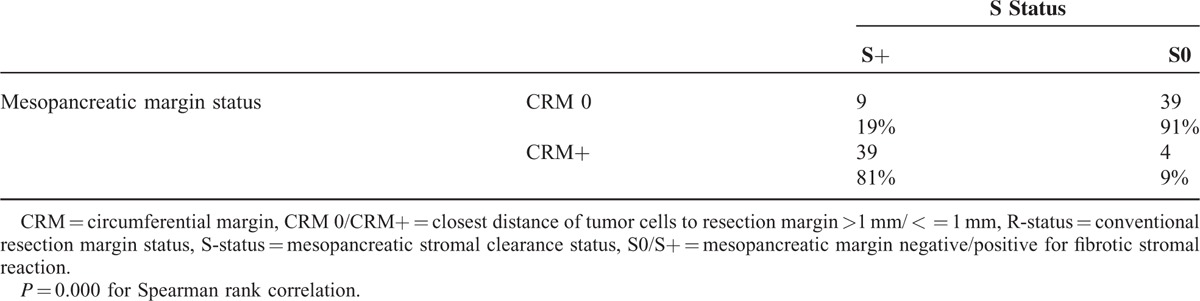
The closest distance between tumor cells and resection margin was measured at the mesopancreatic margin and categorized according to the CRM concept 17 as positive (CRM+) when tumor cells were found within 1 mm from the resection margin. There was a strong correlation between the categories S+ and CRM+, with 81% of the S+ cases also being CRM positive and 91% of the S0 cases being CRM negative (Table 4, P < 0.001 for 2-sided Spearman Rank correlation).
TABLE 4.
Correlation Between S Status and CRM Concept
Prediction of Stromal Clearance Status by Radiographic Parameters
As fibrotic changes in the mesopancreatic fatty tissue can be visualized by stranding in MDCT or MRI, we evaluated a multivariate model for the prediction of mesopancreatic S-status (supplemental Table S2). Nineteen patients had to be excluded from this analysis due to unavailable preoperative imaging sets or insufficient image quality.
In univariate logistic regression analysis, thickness and stranding of the SMA fat sheath were significant predictors of S-status, while stranding of the fat plane between ICV and pancreas reached a statistical trend (P = 0.05). In a multivariate logistic regression model including these 3 parameters, only SMA fat sheath stranding qualified as independent predictor. Cross tabulation analysis disclosed positive and negative predictive values of 77% and 66%, with an overall accuracy of 71% for prediction of S-status by SMA fat sheath stranding (supplemental Table S2).
DISCUSSION
Prognosis of PDAC remains poor even in patients with radical surgical resection, due to local and systemic recurrence.2,3,32 Several hypotheses are usually given to explain these clinical observations. On the one hand, PDAC is supposed to have an intrinsic aggressive biology featuring highly invasive cancer cells,1 discontinuous growth,33 perineural spread,34 as well as high metastatic potency.8 Nevertheless, data to support the a priori assumption that PDAC is intrinsically more aggressive than other carcinomas is very scarce at best.
On the other hand, radical wide surgical resection is anatomically impossible and therefore successful surgical resection has been conventionally defined as the achievement of histopathologically tumor cell free margins (R0 resection).17 Positive resection margins in pancreatoduodenectomy specimen are most frequently found in the retroperitoneal tissue dorsal to the pancreatic head and neck and toward the superior mesenteric artery.14–17 This area has previously been coined “mesopancreas.”10,11 Other authors refer to it as the retroperitoneal, medial, posterior, uncinate, or superior mesenteric artery margin.14–17 We prefer to use the term mesopancreas because it describes well its development and function.10 In our series this margin was routinely marked by the surgeon and was found to be the most critical margin in terms of conventional margin status.
Given the anatomic complexity of pancreatic head resection, it is not surprising that margin status derived from nonstandardized histopathologic workup protocols frequently failed to achieve prognostic value.17 Detection of tumor positive margins according to the few currently standardized protocols essentially relies on 2 measures: extensive specimen workup by serial tissue slicing with resection plain inking and definition of a negative margin by a minimum distance of tumor cells from the inked resection plain. These concepts were developed in analogy to the circumferential margin workup of rectal cancer resection specimens.17 Using these novel protocols, over 80% of pancreatoduodenectomy specimen were found to yield positive margins,17 providing a simple yet important explanation for the notorious failure of surgical therapy.
It remains unclear whether the more extensive circumferential workup by serial tissue slicing or the assessment of the distance between tumor cells and resection margin contributes to this recently increased detection of margin positive resection. The definition of a margin negative resection remains a matter of debate. As a biological rationale for a distance cut-off, the dispersed growth of PDAC has been suggested.17,33 In the absence of data defining a widely accepted cut-off, most authors rely on a minimum tumor cell to margin cut-off of 1 mm, in analogy to the CRM concept in rectal cancer.17
The current study evaluates a clinically and biologically inspired classification of margin assessment in PDAC. Clinical experience shows that a strong fibrotic stromal reaction can often be observed in the peritumoral mesopancreatic tissue, sometimes even necessitating sharp dissection, especially from major blood vessels. Given these clinicopathologic experiences and the apparent prominent role of the desmoplastic stroma in PDAC, we tested the simple mechanistic hypothesis that clearance of the peritumoral fibrotic stroma determines the oncologic outcome of resection.
As a potential drawback, our study was performed retrospectively on the basis of a patient cohort that had not been assessed by 1 of the novel extensive standard workup protocols. Nevertheless, the conventional margin status (R-status) achieved statistically significant influence on overall survival after resection. These results highlight that a focused standard approach, concentrating on cooperation of surgeons and pathologists, results in clinically valid margin assessment. It further confirms the results of other authors 14,35–38 demonstrating the mesopancreatic margin as the clinically most influential of all margins in pancreatoduodenectomy. Only less than 10% of resections were R+ because of isolated involvement of the pancreatic parenchymal margin, and only 2% of other margins were even found to be positive.
While roughly one-third of resections were margin positive (R+) by conventional means, more than half of all cases were retrospectively found to have stromal positive (S+) mesopancreatic resection margins in re-evaluation. Furthermore, only stromal clearance at the mesopancreatic margin (S0 resection) had a very significant and strong positive influence on overall survival after resection. Of note, lymph node ratio was the other independent prognostic parameter in multivariate analysis. S-status additionally provided a clear prognostic subcategorization of the patient group with conventional R0 status. The small subgroup of patients with lymph node negative disease and mesopancreatic stromal clearance displayed a very favorable prognosis rarely observed in PDAC.
The positive correlation of S+ resection with lymph node ratio may suggest that S-status is also related to intrinsic tumor aggressiveness. It might be speculated that more aggressive tumors display more effective lymphatic dissemination on the one hand and more diffuse growth on the other hand, rendering them less amenable to S0 resection. Similar observations have been reported by other authors for the correlation of R-status with lymphangiosis and lymph node metastasis.39,40 Further biologic interpretation remains to be examined. On the basis of our data we cannot decide whether peritumoral fibrotic stroma left in place results in cancer recurrence or whether stromal clearance is just a sensitive surrogate marker of undetected dispersed cancer cells left behind.
Some authors have recently suggested total mesopancreatic resection,41–43 which involves paraaortic lymphadenectomy of stations 16a and 16b as well as circular lymphadenectomy around the superior mesenteric artery.11 However, in the absence of evidence for a survival benefit, but instead increased morbidity with extended lymphadenectomy in randomized trials,44 total mesopancreatic resection is not advocated in the current guidelines of the International Study group for Pancreatic Surgery (ISGPS).44 At our institution, only standard lymphadenectomy was performed during pancreatoduodenectomy, corresponding to the current ISGPS guidelines. This includes en bloc resection of mesopancreatic tissue dorsal to the pancreatic head and neck and to the right side of the superior mesenteric artery.44
Theoretically, in case of macroscopic tumor infiltration, total mesopancreatic resection could result in stroma-negative margins and survival benefit. This hypothesis would have to be tested in a randomized trial. Currently, however, taking into account the data from randomized controlled trials on extended lymphadenectomy,44 we do not feel that our findings advocate routine extended lymphadenectomy/total mesopancreatic resection.
We further demonstrate feasibility of correct preoperative prediction of stromal clearance on the basis of standard cross-sectional imaging in over 70% of patients. This issue has not been assessed for the other novel workup protocols 17,23 and should be validated, ideally in a prospective randomized fashion. According to our data, about half of all patients selected for upfront pancreatic head cancer surgery can be expected to have no stromal clearance from resection. Consequently, measures to down-stage the tumor before resection may be advocated. In current clinical practice, only so-called borderline resectable PDAC is a widely accepted indication for neoadjuvant treatment. However, our radiologic criteria are more stringent than those currently proposed to define borderline resectable PDAC.18,22 In summary, our data may suggest that a redefinition of borderline resectable disease and broader application of neoadjuvant treatment may be necessary to meet the clinical challenge of pancreatic cancer, when the above-mentioned criteria are present. The proposed mesopancreatic stromal clearance status proved to be a very powerful and clinically valid prognostic factor in patients receiving resection of pancreatic head cancer.
Supplementary Material
Acknowledgment
We thank Markus Kuehs and Simon Cigolla for expert technical assistance.
Footnotes
Abbreviations: CRM = circumferential margin, CT = computed tomography, FFPE = formalin fixed paraffin embedded, H&E = hematoxylin-eosin stain, HA = hepatic artery, ICV = inferior caval vein, ISGPS = international study group for pancreatic surgery, MDCT = multiphasic multidetector computed tomography, MRI = magnetic resonance imaging, PDAC = pancreatic ductal adenocarcinoma, PVR = superior mesenteric vein/portal vein resection, R- = conventional margin negative, R+ = conventional margin positive, R-status = conventional resection margin status, S+ = stromal positive mesopancreatic margin, S0 = stromal negative mesopancreatic margin, SMA = superior mesenteric artery, S-status = mesopancreatic stromal clearance status, UICC = Union for International Cancer Control, WHO = World Health Organization.
UFW and T Krauss contributed equally to this work.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Ryan DP, Hong TS, Bardeesy N. Pancreatic Adenocarcinoma. N Engl J Med 2014; 371:1039–1049. [DOI] [PubMed] [Google Scholar]
- 2.Quaresma M, Coleman MP, Rachet B. 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971–2011: a population-based study. Lancet 2014; 385:1206–1218. [DOI] [PubMed] [Google Scholar]
- 3.Sun H, Ma H, Hong G, et al. Survival improvement in patients with pancreatic cancer by decade: a period analysis of the SEER database, 1981-2010. Sci Rep 2014; 4:6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis R, Drebin JA, Callery MP, et al. A contemporary analysis of survival for resected pancreatic ductal adenocarcinoma. HPB 2013; 15:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDowell BD, Chapman CG, Smith BJ, et al. Pancreatectomy predicts improved survival for pancreatic adenocarcinoma: results of an instrumental variable analysis. Ann Surg 2015; 261:740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrone CR, Pieretti-Vanmarcke R, Bloom JP, et al. Pancreatic ductal adenocarcinoma: long-term survival does not equal cure. Surgery 2012; 152 (3 Suppl 1):S43–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen KT-K, Singla S, Papavasiliou P, et al. Patterns of recurrence and outcomes in pancreatic cancer. J Clin Oncol 2013; 31 (Suppl 4; abstr 234.): http://meetinglibrary.asco.org/content/105288-133http://meetinglibrary.asco.org/content/105288-133. Accessed December 30, 2014. [Google Scholar]
- 8.Sugiura T, Uesaka K, Mihara K, et al. Margin status, recurrence pattern, and prognosis after resection of pancreatic cancer. Surgery 2013; 154:1078–1086. [DOI] [PubMed] [Google Scholar]
- 9.Van den Broeck A, Sergeant G, Ectors N, et al. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 2009; 35:600–604. [DOI] [PubMed] [Google Scholar]
- 10.Gockel I, Domeyer M, Wolloscheck T, et al. Resection of the mesopancreas (RMP): a new surgical classification of a known anatomical space. World J Surg Oncol 2007; 5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peparini N. Mesopancreas: a boundless structure, namely the rationale for dissection of the paraaortic area in pancreaticoduodenectomy for pancreatic head carcinoma. World J Gastroenterol 2015; 21:2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agrawal MK, Thakur DS, Somashekar U, et al. Mesopancreas: myth or reality? JOP J Pancreas 2010; 11:230–233. [PubMed] [Google Scholar]
- 13.Bouassida M, Mighri MM, Chtourou MF, et al. Retroportal lamina or mesopancreas? Lessons learned by anatomical and histological study of thirty three cadaveric dissections. Int J Surg Lond Engl 2013; 11:834–836. [DOI] [PubMed] [Google Scholar]
- 14.Jamieson NB, Foulis AK, Oien KA, et al. Positive mobilization margins alone do not influence survival following pancreatico-duodenectomy for pancreatic ductal adenocarcinoma. Ann Surg 2010; 251:1003–1010. [DOI] [PubMed] [Google Scholar]
- 15.Konstantinidis IT, Warshaw AL, Allen JN, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg 2013; 257:731–736. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Katz MH, Lee SM, et al. Superior mesenteric artery margin of posttherapy pancreaticoduodenectomy and prognosis in patients with pancreatic ductal adenocarcinoma. Am J Surg Pathol 2015; 39:1395–1403. [DOI] [PubMed] [Google Scholar]
- 17.Verbeke CS. Resection margins in pancreatic cancer. Surg Clin North Am 2013; 93:647–662. [DOI] [PubMed] [Google Scholar]
- 18.Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014; 155:977–988. [DOI] [PubMed] [Google Scholar]
- 19.Castleberry AW, White RR, De La Fuente SG, et al. The impact of vascular resection on early postoperative outcomes after pancreaticoduodenectomy: an analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Ann Surg Oncol 2012; 19:4068–4077. [DOI] [PubMed] [Google Scholar]
- 20.Chua TC, Saxena A. Extended pancreaticoduodenectomy with vascular resection for pancreatic cancer: a systematic review. J Gastrointest Surg Off J Soc Surg Aliment Tract 2010; 14:1442–1452. [DOI] [PubMed] [Google Scholar]
- 21.Hartwig W, Vollmer CM, Fingerhut A, et al. Extended pancreatectomy in pancreatic ductal adenocarcinoma: definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS). Surgery 2014; 156:1–14. [DOI] [PubMed] [Google Scholar]
- 22.Katz MHG, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol 2013; 20:2787–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denecke T, Grieser C, Neuhaus P, et al. Radiologic resectability assessment in pancreatic cancer. RöFo Fortschritte Auf Dem Geb Röntgenstrahlen Nukl 2014; 186:23–29. [DOI] [PubMed] [Google Scholar]
- 24.Katz MHG, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012; 118:5749–5756. [DOI] [PubMed] [Google Scholar]
- 25.Apte MV, Xu Z, Pothula S, et al. Pancreatic cancer: the microenvironment needs attention too!. Pancreatol Off J Int Assoc Pancreatol IAP Al 2015; 15 (4 Suppl):S32–S38. [DOI] [PubMed] [Google Scholar]
- 26.Neesse A, Algül H, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut 2015; 64:1476–1484. [DOI] [PubMed] [Google Scholar]
- 27.Moffitt RA, Marayati R, Flate EL, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet 2015; 47:1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014; 25:735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of Tumors of the Digestive System. 4th edLyon, France: International Agency for Research in Cancer; 2010. [Google Scholar]
- 30.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7th edAuflage: Wiley-Blackwell; 2011. [Google Scholar]
- 31.Seufferlein T, Porzner M, Becker T, et al. [S3-guideline exocrine pancreatic cancer]. Z Für Gastroenterol 2013; 51:1395–1440. [DOI] [PubMed] [Google Scholar]
- 32.Lepage C, Capocaccia R, Hackl M, et al. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999-2007: results of EUROCARE-5. Eur J Cancer 2015; 51:2169–2178. [DOI] [PubMed] [Google Scholar]
- 33.Verbeke CS, Knapp J, Gladhaug IP. Tumour growth is more dispersed in pancreatic head cancers than in rectal cancer: implications for resection margin assessment. Histopathology 2011; 59:1111–1121. [DOI] [PubMed] [Google Scholar]
- 34.Liebl F, Demir IE, Mayer K, et al. The impact of neural invasion severity in gastrointestinal malignancies: a clinicopathological study. Ann Surg 2014; 260:900–908. [DOI] [PubMed] [Google Scholar]
- 35.Gaedcke J, Gunawan B, Grade M, et al. The mesopancreas is the primary site for R1 resection in pancreatic head cancer: relevance for clinical trials. Langenbecks Arch Surg 2010; 395:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gnerlich JL, Luka SR, Deshpande AD, et al. Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch Surg Chic 2012; 147:753–760. [DOI] [PubMed] [Google Scholar]
- 37.Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg 2007; 246:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Frampton AE, Cohen P, et al. Tumor infiltration in the medial resection margin predicts survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Gastrointest Surg Off J Soc Surg Aliment Tract 2012; 16:1875–1882. [DOI] [PubMed] [Google Scholar]
- 39.Kimbrough CW, St Hill CR, Martin RCG, et al. Tumor-positive resection margins reflect an aggressive tumor biology in pancreatic cancer. J Surg Oncol 2013; 107:602–607. [DOI] [PubMed] [Google Scholar]
- 40.Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg 2001; 234:758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adham M, Singhirunnusorn J. Surgical technique and results of total mesopancreas excision (TMpE) in pancreatic tumors. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 2012; 38:340–345. [DOI] [PubMed] [Google Scholar]
- 42.Aimoto T, Mizutani S, Kawano Y, et al. Left posterior approach pancreaticoduodenectomy with total mesopancreas excision and circumferential lymphadenectomy around the superior mesenteric artery for pancreatic head carcinoma. J Nippon Med Sch Nippon Ika Daigaku Zasshi 2013; 80:438–445. [DOI] [PubMed] [Google Scholar]
- 43.Kawabata Y, Tanaka T, Nishi T, et al. Appraisal of a total meso-pancreatoduodenum excision with pancreaticoduodenectomy for pancreatic head carcinoma. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 2012; 38:574–579. [DOI] [PubMed] [Google Scholar]
- 44.Tol JAMG, Gouma DJ, Bassi C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014; 156:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


