Abstract
Dysregulation of inflammatory cytokines and liver injury are associated with the pathogenesis of sepsis. Xuebijing injection, a Chinese herbal medicine, has been used in the treatment of sepsis and can contribute to the improvement of patients' health. However, the underlying molecular mechanisms are not yet clearly illuminated. In the present study, a septic rat model with liver injury was established by the cecal ligation and puncture (CLP) method. Histological alterations to the liver, activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), levels of inflammatory cytokine secretion and the expression of suppressors of cytokine signaling 1 (SOCS-1) in the CLP model rats with and without Xuebijing treatment were determined. The results showed that Xuebijing injection ameliorated the pathological changes in liver tissues caused by sepsis, and reduced the sepsis-induced elevation in serum ALT and AST levels. Furthermore, Xuebijing injection markedly downregulated the expression of tumor necrosis factor α and interleukin (IL)-6, and upregulated the expression of IL-10. More importantly, SOCS1 expression levels at the protein and mRNA levels were further increased by Xuebijing. These findings demonstrate that Xuebijing injection can significantly alleviate liver injury in CLP-induced septic rats via the regulation of inflammatory cytokine secretion and the promotion of SOCS1 expression. The protective effects of Xuebijing injection suggest its therapeutic potential in the treatment of CLP-induced liver injury.
Keywords: Xuebijing injection, sepsis, SOCS-1
Introduction
Sepsis is a serious systemic inflammatory response syndrome (SIRS) caused by infection with bacteria, fungi, viruses or parasites (1). The pathogenesis of sepsis is associated with disorder of whole-body inflammatory reaction networks (2). When the immune system becomes overactivated in response to an existing infection, the inflammatory response may lead to dysfunction of vital organs, such as the lung, heart, kidney and liver (3). The liver plays a critical role in the host defense mechanism (4), and is one of the organs most frequently affected by organ dysfunction in sepsis (5). The liver modulates inflammatory processes, and produces and releases high amounts of various cytokines (6), which play an important role in the regulation and transduction of inflammatory signals (7). During sepsis, pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α and interleukin (IL)-6, have a crucial effect on the initiation of the inflammatory process (8). Anti-inflammatory cytokines, such as IL-10, are also found to be extensively released in septic patients (9). Moreover, cytokines mediate and amplify inflammatory signals through the Janus kinase-signal transducer and activator of transcription JAK-STAT pathway in a number of diseases (10). A recent study has revealed that suppressors of cytokine signaling (SOCS) are able to suppress cytokine-mediated inflammatory signaling as feedback regulators in the JAK-STAT pathway (11). SOCS-1, a member of the SOCS family, has been experimentally demonstrated to be effective in reducing the inflammatory response in inflammatory diseases (12,13). Mice deficient in SOCS-1 have exhibited liver degeneration and lymphoid deficiencies (14). Furthermore, a previous study has shown that sepsis induces SOCS-1 expression in rat liver and muscle (15), and it has been recognized that SOCS1 plays an important role in LPS-induced liver injury. Therefore, SOCS-1 may be involved in the pathogenesis of liver injury in cecal ligation and puncture (CLP)-induced septic rats.
Xuebijing injection is a newly developed Chinese herbal injection consisting of Chuangxiong Rhizoma (the dried rhizome of Ligusticum chuanxiong Hort.), Paeoniae Radix Rubra (the dried roots of Paeonia lactiflora Pall.), Salviae Miltiorrhizae Radix et Rhizoma (the dried roots and rhizome of Salvia miltiorrhiza Bge.) and Carthami Flos (the dried flower of Carthamus tinctorius L.). It has anti-inflammatory and anti-endotoxic effects in the treatment of sepsis (16). Experimental and clinical studies have revealed that Xuebijing injection is effective for the treatment of SIRS, pyemia and multiple organ dysfunction syndrome (17–19). In addition, Xuebijing injection has been formally approved by the State Food and Drug Administration of China for use in clinical practice and is widely used to treat sepsis in China. However, the underlying molecular mechanisms of Xuebijing injection remain unclear. Thus, the aim of the present study was to investigate the effect of Xuebijing injection on liver injury in CLP-induced septic rats and to explore the underlying mechanism. In particular, the study aimed to focus on the effect of Xuebijing injection on hepatic inflammatory cytokine secretion and SOCS1 expression in rats with sepsis-induced liver injury.
Materials and methods
Chemicals and antibodies
Xuebijing injection was supplied by Tianjin Chase Sun Pharmaceutical Co., Ltd. (Tianjin, China; lot no. Z20070410). Antibodies targeting SOCS-1 (sc-9021) and β-actin (sc-130656) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody (RPN4301) for the western blotting assay was purchased from Amersham (GE Healthcare Life Sciences, Piscataway, NJ, USA). Enzyme-linked immunosorbent assay (ELISA) kits for TNF-α, IL-6 and IL-10 were purchased from R&D Systems, Inc. (Minneapolis, MN, USA). The ABI StepOnePlus Real-Time PCR System was purchased from Applied Biosystems (Thermo Fisher Scientific, Inc., Waltham, MA, USA). All other chemicals used were of analytical grade.
Animals
Male Sprague-Dawley (SD) rats, weighing 240–280 g, were provided by the Experimental Animal Center of Nantong University (Nantong, China) and housed in a temperature- and humidity-controlled environment with a 12-h light-dark cycle. The animals had access to food and water ad libitum. The study protocol was approved by the ethics committee (Institutional Review Board) of Nantong University.
Animal model and treatment
A total of 72 rats were divided into three experimental groups: Control group, CLP group and Xuebijing injection-treated (XT) group. The rats in the latter group were treated with 10 mg/kg Xuebijing injection, intraperitoneally, i.p. Each group contained 24 rats. The septic rat model was induced by CLP as previously described (20). Briefly, all rats underwent fasting for 12 h and water deprivation for 6 h prior to undergoing CLP surgery. Following pentobarbital sodium anesthesia (45 mg/kg, i.p.), the abdominal region was disinfected with iodophor and a 2-cm incision was made, the cecum was then gently isolated with tight ligation and punctured twice with an 18-gauge needle. Thereafter, the cecum was repositioned, and the abdomen was subsequently closed. For the control group alone, rats underwent the same surgical procedures, but the cecum was neither ligated nor punctured. Saline (50 ml/kg body weight) was administered subcutaneously to the rats for fluid resuscitation. For the TX group, rats received an intraperitoneal administration of Xuebijing injection (10 ml/kg body weight) twice per day after CLP surgery for 3 consecutive days. Rats in the control and CLP groups were administered equal volumes of saline instead.
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity measurements
To measure ALT and AST activities, 5-ml blood samples were obtained from the aorta abdominalis of rats in different groups at 6, 12, 24 and 24 h after CLP surgery. The ALT and AST activities were then measured using a Hitachi 7600-020 Automatic Analyzer (Hitachi High-Technologies Corporation, Tokyo, Japan).
ELISA analysis of TNF-α, IL-6, and IL-10
Homogenized liver samples (0.5–1 g; 10%) from the different groups, sampled at 6, 12, 24 and 24 h after CLP surgery, were centrifuged at 1,500 × g for 15 min at 4°C) to obtain a supernatant. The amounts of TNF-α, IL-6 and IL-10 in the liver tissue homogenates were analyzed using commercial ELISA kits specific for rats according to the manufacturer's protocol. Cytokine levels are expressed in units of pg/ml.
Histological examination
The liver tissues from rats in each group were collected at 6 and 48 h after CLP surgery. The tissue samples were fixed in 10% formalin solution, embedded in paraffin, and sectioned. The tissue sections were then stained with hematoxylin and eosin reagent according to standard protocols and observed using light microscopy.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
To determine the mRNA level of SOCS-1, RT-qPCR analysis was performed. Briefly, liver tissues samples (50–100 mg) were collected from rats in the various groups at 6, 12, 24 and 48 h after surgery. Total RNA was then extracted from homogenized liver samples using TRIzol reagent (Gibco Life Technologies; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Total RNAs were treated with DNase I (Fermentas; Thermo Fisher Scientific, Inc.) to remove any contaminating DNA at 37°C for 30 min, followed by deactivation at 65°C for 10 min. The RNA was then reverse transcribed into cDNA using an M-MLV reverse transcription kit (Takara Biotechnology Co., Ltd., Dalian, China) according to the manufacturer's protocol with the ABI StepOnePlus Real-Time PCR system. Following the reverse transcription, fluorescence qPCR was performed using SYBR Green I dye (Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the manufacturer's protocol. The following primers were used: SOCS-1 forward, 5′-CCGCTCCCACTCTGATTACC-3′ and reverse, 5′-CCCGAAGCCATCTTCACG-3′; β-actin forward, 5′-CGGTTCCGATGCCCTGAGGCACTT-3′ and reverse, 5′-CGTCACACTTCATGATGGAATTGA-3′. The samples for RT-qPCR analysis were evaluated using a single predominant peak as a quality control. The comparative Ct (2−∆∆Ct) method (21) was used to analyze the relative expression levels of SOCS1 mRNA, which were normalized to β-actin.
Western blot analysis
Western blot analysis was performed using a similar method to that previously described (22). Briefly, homogenized liver tissues from different groups, sampled at 12 and 48 h, were centrifuged at 13,000 × g for 30 min at 4°C to generate a supernatant containing the extracted protein. The protein concentration was measured using a bicinchoninic acid (BCA) protein assay kit as previously described (23,24). A 50 µg portion of each sample was electrophoresed on polyacrylamide gel (10%) and transferred onto a polyvinylidene difluoride membrane (EMD Millipore, Bedford, MA, USA). After blocking with blocking buffer, the blots were incubated 2 h at 4°C with diluted primary antibodies against SOCS-1 (1:200). The membrane was then washed three times in Tris-buffered saline with Tween-20 (0.1%) and incubated with HRP-conjugated anti-rabbit IgG antibody (dilution, 1:2,500) for 1.5 h at room temperature. The membrane was again washed three times for 10 min each time, and finally the immunoreactive proteins were detected using an enhanced chemiluminescence Western blotting detection kit. The β-actin protein served as an internal control.
Statistical analysis
The data were analyzed using STATA version 10.0 (StataCorp LP, College Station, TX, USA). One-way analysis of variance (ANOVA) was used to analyze the significant differences among different groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Xuebijing injection ameliorates sepsis-induced histological liver injury in rats
Hematoxylin and eosin staining clearly revealed liver injury characterized by edema, ballooning degeneration, fatty degeneration, thrombosis and infiltration of inflammatory cells in the liver tissues at 6 h after CLP surgery (Fig. 1). When examined at 48 h after CLP surgery, hepatic cords were largely broken and all liver injury symptoms become much more severe. However, these histological alterations induced by CLP surgery were ameliorated by Xuebijing injection treatment (Fig. 1).
Figure 1.
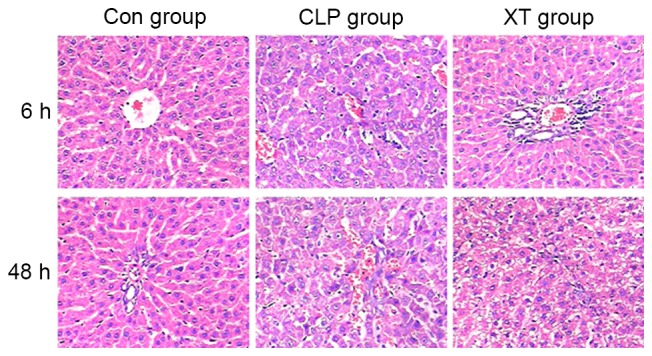
Effects of Xuebijing injection on CLP-induced histological liver injury in CLP-induced septic rats. Con group, control group; CLP group, cecal ligation and puncture group; XT group, CLP surgery plus Xuebijing injection treatment. Liver sections with hematoxylin and eosin staining (magnification, ×200).
Xuebijing injection decreases ALT and AST levels in rats with septic liver injury
ALT and AST levels of rats from different groups were measured at 6, 12, 24 and 24 h after CLP surgery. Sepsis induced an increase of serum ALT and AST levels in rats in comparison with those in the control group (P<0.05), which was indicative of liver injury. However, as shown in Fig. 2, treatment with Xuebijing injection significantly decreased ALT and AST levels in this model (P<0.05).
Figure 2.
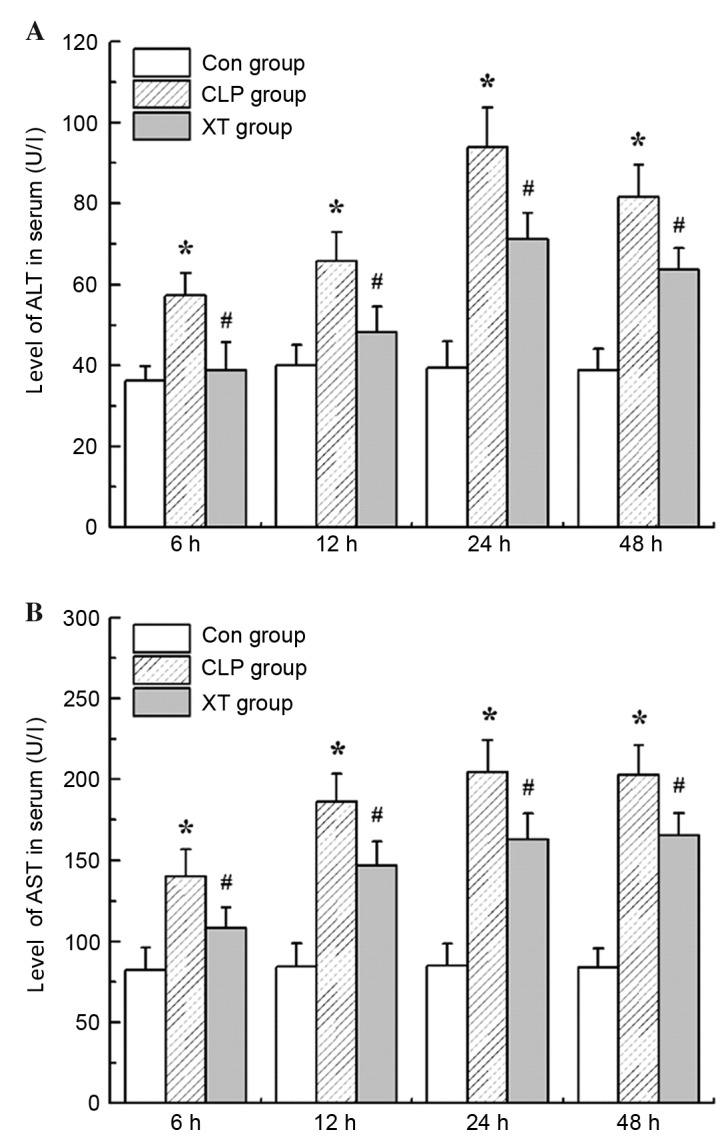
Effects of Xuebijing injection on the levels of (A) ALT and (B) AST in CLP-induced septic rats. ALT, alanine aminotransferase; AST, aspartate aminotransferase; Con group, control group; CLP group, cecal ligation and puncture group; XT group, CLP surgery plus Xuebijing injection treatment. Data are expressed as the mean ± standard deviation (n=6). *P<0.05 vs. the Con group; #P<0.05 vs. the CLP group.
Xuebijing injection regulates the secretion of inflammatory cytokines in CLP-induced septic rats
Sepsis is characterized by the uncontrollable release of pro-inflammatory and anti-inflammatory cytokines (25). In the present study, two pro-inflammatory cytokines, TNF-α and IL-6, and one anti-inflammatory cytokine, IL-10 were detected at 6, 12, 24 and 24 h after CLP surgery using ELISA kits. The results in Fig. 3 show that hepatic TNF-α, IL-6 and IL-10 levels were significantly elevated in CLP-induced septic rats in comparison with those in the control group (P<0.05). However, Xuebijing injection treatment significantly downregulated hepatic TNF-α and IL-6 levels, while upregulating hepatic IL-10 levels in CLP-induced septic rats (P<0.05 for each).
Figure 3.
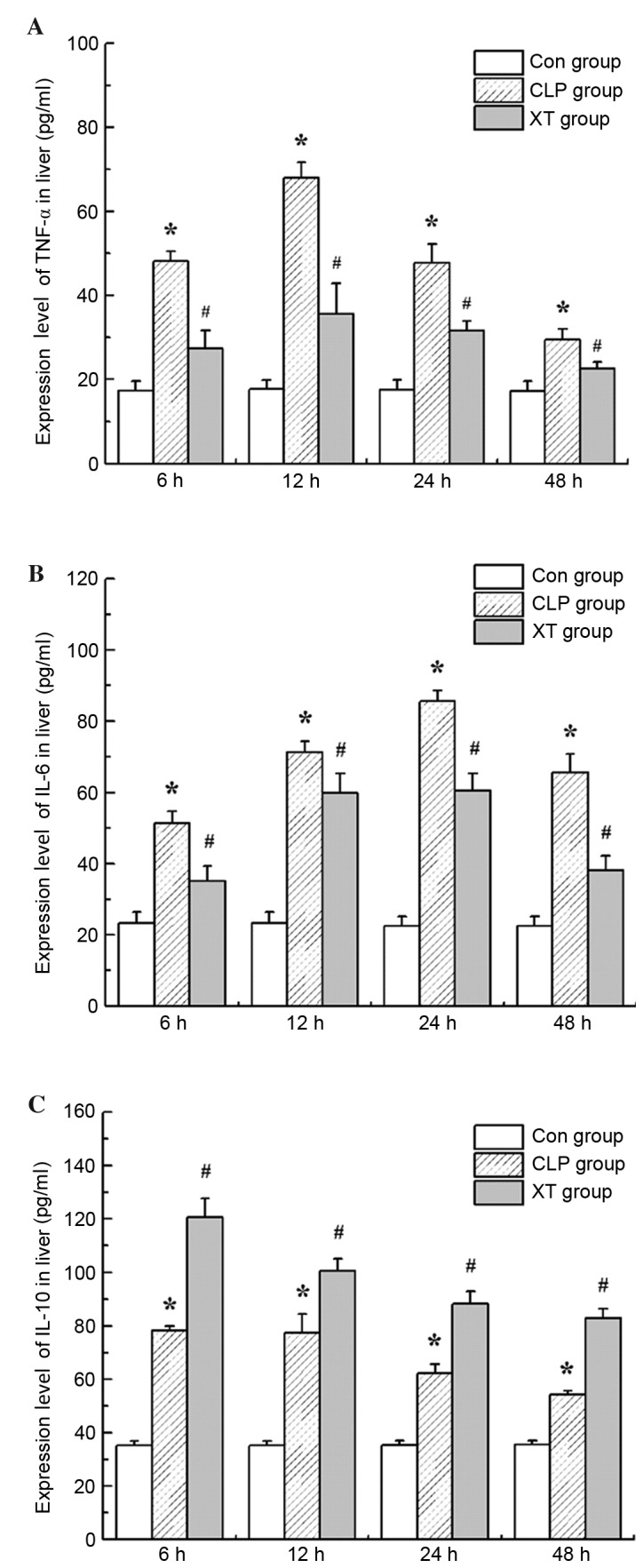
Effects of Xuebijing injection on the secretion of inflammatory cytokines in CLP-induced septic rats. Expression levels of (A) TNF-α, (B) IL-6 and (C) IL-10 were determined. TNF, tumor necrosis factor; IL, interleukin; Con group, control group; CLP group, cecal ligation and puncture group; XT group, CLP surgery plus Xuebijing injection treatment. Values are expressed as the mean ± standard deviation (n=6). *P<0.05 vs. the Con group; #P<0.05 vs. the CLP group.
Xuebijing injection elevates the expression of SOCS-1 in CLP-induced septic rats
Numerous studies have found that SOCS proteins are negative-feedback regulators in the JAK-STAT pathway (11). In the present study, the expression of SOCS-1 was analyzed by RT-qPCR and western blotting. As shown in Fig. 4, elevated SOCS-1 mRNA and protein expression levels were observed in CLP-induced septic rats (P<0.05 for mRNA), and the increases were further increased by the Xuebijing injection treatment (Fig. 4).
Figure 4.
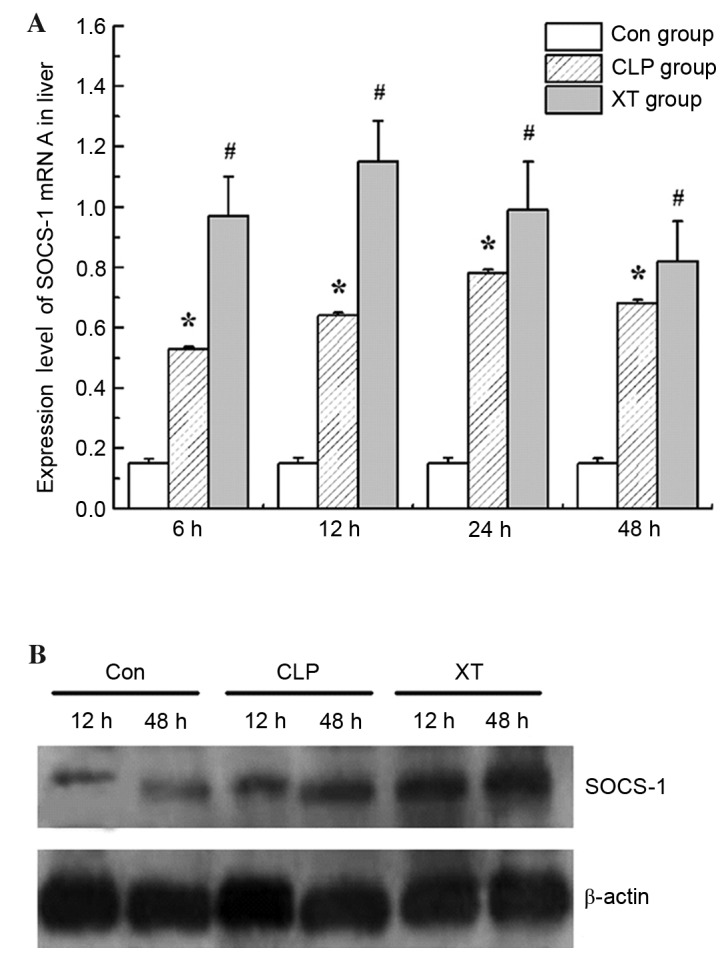
Effects of Xuebijing injection on the expression of SOCS-1 in CLP-induced septic rats. (A) SOCS-1 mRNA expression levels determined by reverse transcription-quantitative polymerase chain reaction. Values are expressed as the mean ± standard deviation of 6 rats in each group. *P<0.05 vs. the Con group; #P<0.05 vs. the CLP group. (B) A representative Western blot from three experiments is shown; β-actin was used as a loading control. SOCS, suppressors of cytokine signaling; Con group, control group; CLP group, cecal ligation and puncture surgery group; XT group, CLP surgery plus Xuebijing injection treatment.
Discussion
In the present study, it was demonstrated that Xuebijing injection treatment attenuated liver injury induced by sepsis. Furthermore, the results showed that Xuebijing injection treatment downregulated TNF-α and IL-6 levels, and upregulated IL-10 levels in the livers of septic rats, which was associated with the expression of SOCS1. Therefore, the present study suggests that Xuebijing injection protects against sepsis-induced liver injury by regulating the secretion of inflammatory cytokines and increasing the expression of SOCS1 in the livers of septic rats.
A previous clinicopathological study reported that patients dying from sepsis typically had inflammation and necrosis in the liver; increased levels of markers of abnormal liver biochemistry, including ALT and AST, were also observed in septic patients prior to death (26). In the present study, CLP-induced sepsis caused an inflammatory response and damage in the liver, which was characterized both histologically and biochemically. The treatment with Xuebijing injection ameliorated the pathological changes in liver tissues caused by sepsis, and also decreased ALT and AST levels in the rats with septic liver injury.
Evidence indicates that inflammatory cytokines play an important role in sepsis; pro-inflammatory cytokines and anti-inflammatory cytokines counteract each other and reach an immunological equilibrium, which leads to various types of tissue damage and organ injury (8). In the present study, elevated TNF-α, IL-6 and IL-10 levels were observed in the livers of rats in the CLP group in comparison with those in the control group, which is consistent with previous studies (27,28). Moreover, Xuebijing injection has been found to reduce the secretion of TNF-α and IL-6 by Kupffer cells in livers of rats with heart stroke (29), and promote IL-10 expression in rabbits with oleic acid-induced acute lung injury (30). The present study found that Xuebijing injection significantly downregulated pro-inflammatory cytokine levels, specifically TNF-α and IL-6, while upregulating the levels of the anti-inflammatory cytokine IL-10 in rats with septic liver injury. These data suggest that the protective effects of Xuebijing injection on septic liver injury may occur via the regulation of the secretion of inflammatory cytokines.
Cytokine signaling induces SOCS1, a protein that can inhibit the signaling pathways that stimulated its production (31,32). SOCS-1 is a negative-feedback regulator not only in the JAK-STAT pathway but also in the lipopolysaccharide-nuclear factor-κB (LPS-NF-κB) signaling pathway (33). SOCS-1 has been found to inhibit cytokine signaling in all systems tested, including TNF-α and IL-6 (34). TNF-α-mediated inflammatory signals can be transduced through the JAK-STAT pathway (35,36). The results of the present study demonstrated that SOCS-1 expression was upregulated by Xuebijing injection, which corresponded with its relieving effects on inflammatory response and liver injury. Collectively, those results indicate that Xuebijing injection ameliorates liver injury in CLP-induced septic rats by promoting SOCS-1 expression at the protein and mRNA levels.
In summary, the present study demonstrated that Xuebijing injection can effectively alleviate liver injury in a rat model of CLP-induced sepsis. The protective effects of Xuebijing injection on septic liver injury are associated with the regulation of inflammatory cytokine secretions and the promotion of SOCS1 expression. This study provides valuable insights into the role of Xuebijing injection in septic liver injury, and reveals its therapeutic potential in CLP-induced liver injury treatment.
Acknowledgements
This study was supported by grants from the China Postdoctoral Science Foundation (2013M541707), Jiangsu Postdoctoral Science Foundation, Natural Science Fund Project in Zhejiang Province (Y2100917) and Nantong Science and Technology Project (HS149095, HS149137 and HS2014075).
Glossary
Abbreviations
- SOCS
suppressors of cytokine signaling
- TNF-α
tumor necrosis factor-α
- NF-κB
nuclear factor κB
- IL-6
interleukin-6
- IL-10
interleukin-10
- SIRS
systemic inflammatory response syndrome
- CLP
cecal ligation and puncture
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- RT-qPCR
reverse transcription-quantitative polymerase chain reaction
- ELISA
enzyme-linked immunosorbent assay
- JAK-STAT
janus kinase-signal transducer and activator of transcription
- LPS-NF-κB
lipopolysaccharide-nuclear factor-κB
References
- 1.Jones AE, Puskarich MA. The surviving sepsis campaign guidelines 2012: Update for emergency physicians. Ann Emerg Med. 2014;63:35–47. doi: 10.1016/j.annemergmed.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 3.Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med. 2007;35:2408–2416. doi: 10.1097/01.CCM.0000282072.56245.91. [DOI] [PubMed] [Google Scholar]
- 4.Spapen H. Liver perfusion in sepsis, septic shock, and multiorgan failure. Anat Rec (Hoboken) 2008;291:714–720. doi: 10.1002/ar.20646. [DOI] [PubMed] [Google Scholar]
- 5.Regel G, Grotz M, Weltner T, Sturm JA, Tscherne H. Pattern of organ failure following severe trauma. World J Surg. 1996;20:422–429. doi: 10.1007/s002689900067. [DOI] [PubMed] [Google Scholar]
- 6.Dhainaut JF, Marin N, Mignon A, Vinsonneau C. Hepatic response to sepsis: Interaction between coagulation and inflammatory processes. Crit Care Med. 2001;29(Suppl 7):S42–S47. doi: 10.1097/00003246-200107001-00016. [DOI] [PubMed] [Google Scholar]
- 7.Zhang JM, An J. Cytokines, inflammation and pain. Int Anesthesiol Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: Potent immunoregulators and potential therapeutic targets-an updated view. Mediators Inflamm. 2013;2013:165974. doi: 10.1155/2013/165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman G, Jankowski S, Marchant A, Goldman M, Kahn RJ, Vincent JL. Blood interleukin 10 levels parallel the severity of septic shock. J Crit Care. 1997;12:183–187. doi: 10.1016/S0883-9441(97)90030-7. [DOI] [PubMed] [Google Scholar]
- 10.Villarino AV, Kanno Y, Ferdinand JR, O'Shea JJ. Mechanisms of Jak/STAT signaling in immunity and disease. J Immunol. 2015;194:21–27. doi: 10.4049/jimmunol.1401867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander WS. Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol. 2002;2:410–416. doi: 10.1038/nri818. [DOI] [PubMed] [Google Scholar]
- 12.Yamana J, Yamamura M, Okamoto A, Aita T, Iwahashi M, Sunahori K, Makino H. Resistance to IL-10 inhibition of interferon gamma production and expression of suppressor of cytokine signaling 1 in CD4+T cells from patients with rheumatoid arthritis. Arthritis Res Ther. 2004;6:R567–R577. doi: 10.1186/ar1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan PJ, Lawlor KE, Alexander WS, Wicks IP. Suppressor of cytokine signaling-1 regulates acute inflammatory arthritis and T cell activation. J Clin Invest. 2003;111:915–924. doi: 10.1172/JCI16156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starr R, Metcalf D, Elefanty AG, Brysha M, Willson TA, Nicola NA, Hilton DJ, Alexander WS. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc Natl Acad Sci USA. 1998;95:14395–14399. doi: 10.1073/pnas.95.24.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson TS, O'Leary M, Justice SK, Maamra M, Zarkesh-Esfahani SH, Furlanetto R, Preedy VR, Hinds CJ, El Nahas AM, Ross RJ. Differential expression of suppressors of cytokine signalling genes in response to nutrition and growth hormone in the septic rat. J Endocrinol. 2001;169:409–415. doi: 10.1677/joe.0.1690409. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, Dai G, Hu J, Rong A, Lv J, Su L, Wu X. Discovery of Xuebijing injection exhibiting protective efficacy on sepsis by inhibiting the expression of HMGB1 in septic rat model designed by cecal ligation and puncture. Am J Ther. 2015 Aug 25; doi: 10.1097/MJT.0000000000000296. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 17.Yin Q, Li C. Treatment effects of Xuebijing injection in severe septic patients with disseminated intravascular coagulation. Evid Based Complement Alternat Med. 2014;2014:949254. doi: 10.1155/2014/949254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He XD, Wang Y, Wu Q, Wang HX, Chen ZD, Zheng RS, Wang ZS, Wang JB, Yang Y. Xuebijing protects rats from sepsis challenged with Acinetobacter baumannii by promoting annexin A1 expression and inhibiting proinflammatory cytokines secretion. Evid Based Complement Alternat Med. 2013;2013:804940. doi: 10.1155/2013/804940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu MW, Wang YH, Qian CY, Li H. Xuebijing exerts protective effects on lung permeability leakage and lung injury by upregulating Toll-interacting protein expression in rats with sepsis. Int J Mol Med. 2014;34:1492–1504. doi: 10.3892/ijmm.2014.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Yu B, Xu P, Zhao Z, Cai J, Sternberg P, Chen Y. Subcellular distribution and activity of mechanistic target of rapamycin in aged retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2014;55:8638–8650. doi: 10.1167/iovs.14-14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y, Dou SX, Ren H, Wang PY, Zhang XD, Qian M, Pan BY, Xi XG. Evidence for a functional dimeric form of the PcrA helicase in DNA unwinding. Nucleic Acids Res. 2008;36:1976–1989. doi: 10.1093/nar/gkm1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Dou SX, Xu YN, Bazeille N, Wang PY, Rigolet P, Xu HQ, Xi XG. Kinetic mechanism of DNA unwinding by the BLM helicase core and molecular basis for its low processivity. Biochemistry. 2010;49:656–668. doi: 10.1021/bi901459c. [DOI] [PubMed] [Google Scholar]
- 25.Adrie C, Pinsky MR. The inflammatory balance in human sepsis. Intensive Care Med. 2000;26:364–375. doi: 10.1007/s001340051169. [DOI] [PubMed] [Google Scholar]
- 26.Koskinas J, Gomatos IP, Tiniakos DG, Memos N, Boutsikou M, Garatzioti A, Archimandritis A, Betrosian A. Liver histology in ICU patients dying from sepsis: A clinico-pathological study. World J Gastroenterol. 2008;14:1389–1393. doi: 10.3748/wjg.14.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu W, Bao R, Fan X, Tao T, Zhu J, Wang J, Li J, Bo L, Deng X. PD-L1 blockade attenuated sepsis-induced liver injury in a mouse cecal ligation and puncture model. Mediat Inflamm. 2013;2013:361501. doi: 10.1155/2013/361501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Wang W, Fang H, Yang Y, Li X, He J, Jiang X, Wang W, Liu S, Hu J, et al. GSK-3β inhibition attenuates CLP-induced liver injury by reducing inflammation and hepatic cell apoptosis. Mediators Inflamm. 2014;2014:629507. doi: 10.1155/2014/629507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Tong H, Zhang X, Tang L, Pan Z, Liu Z, Duan P, Su L. Xuebijing injection alleviates liver injury by inhibiting secretory function of Kupffer cells in heat stroke rats. J Tradit Chin Med. 2013;33:243–249. doi: 10.1016/S0254-6272(13)60133-8. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Ji M, Wang L, Chen L, Li J. Xuebijing injection improves the respiratory function in rabbits with oleic acid-induced acute lung injury by inhibiting IL-6 expression and promoting IL-10 expression at the protein and mRNA levels. Exp Ther Med. 2014;8:1593–1598. doi: 10.3892/etm.2014.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung CS, Chen Y, Grutkoski PS, Doughty L, Ayala A. SOCS-1 is a central mediator of steroid-increased thymocyte apoptosis and decreased survival following sepsis. Apoptosis. 2007;12:1143–1153. doi: 10.1007/s10495-007-0059-7. [DOI] [PubMed] [Google Scholar]
- 32.Davey G, Heath W, Starr R. SOCS1: A potent and multifaceted regulator of cytokines and cell-mediated inflammation. Tissue Antigens. 2006;67:1–9. doi: 10.1111/j.1399-0039.2005.00532.x. [DOI] [PubMed] [Google Scholar]
- 33.Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003;3:900–911. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- 34.Fujimoto M, Naka T. Regulation of cytokine signaling by SOCS family molecules. Trends Immunol. 2003;24:659–666. doi: 10.1016/j.it.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Guo D, Dunbar JD, Yang CH, Pfeffer LM, Donner DB. Induction of Jak/STAT signaling by activation of the type 1 TNF receptor. J Immunol. 1998;160:2742–2750. [PubMed] [Google Scholar]
- 36.Miscia S, Marchisio M, Grilli A, Di Valerio V, Centurione L, Sabatino G, Garaci F, Zauli G, Bonvini E, Di Baldassarre A. Tumor necrosis factor alpha (TNF-alpha) activates Jak1/Stat3-Stat5B signaling through TNFR-1 in human B cells. Cell Growth Differ. 2002;13:13–18. [PubMed] [Google Scholar]


