Abstract
To systematically assess the effect of metformin on colorectal cancer (CRC) risk and mortality in type 2 diabetes mellitus (T2DM) patients.
We conducted a systematic search of PubMed, Web of Science, and the Cochrane Library databases for relevant articles before August 2015. Two investigators identified and extracted data independently. We adopted adjusted estimates to calculate summary estimates with 95% confidence interval (CI) using either a fixed-effects or a random-effects model. Subgroup and sensitivity analyses were conducted to evaluate the robustness of the pooled results. The risk of publication bias was assessed by examining funnel plot asymmetry as well as Begg test and Egger test.
Fifteen studies on CRC incidence and 6 studies on CRC survival were finally included in our meta-analysis. The pooled odds ratio (OR) of observational studies illustrated that a slight 10% reduction of CRC incidence was associated with metformin use (OR = 0.90, 95% CI: 0.85–0.96). Furthermore, the pooled hazard ratio (HR) revealed an improved survival outcome for metformin users in CRC patients compared to nonusers (HR = 0.68, 95% CI: 0.58–081). There was no publication bias across studies.
Our meta-analysis demonstrated that metformin therapy could slightly reduce CRC incidence and moderately improve the survival outcomes in patients with T2DM. More prospective studies are warranted to certify this protective association.
INTRODUCTION
Colorectal cancer (CRC) is the second most commonly prevalent cancer in males and the third most commonly malignant disease in females in America.1 It is a leading cause of cancer-related deaths in America2, Europe3, and Asia.4 Regular screening with colonoscopy in high-risk population is a preferred approach recommended by the American Cancer Society (ACS).5 Given limitations of screening examinations, unfortunately, there is a great interest on exploring chemopreventive drugs to reduce the huge burden of CRC.
Metformin, as a first-line treatment for type 2 diabetes mellitus (T2DM), is reported reducing the incidence of many cancers, including CRC.6,7 Previous studies suggested that T2DM is closely related with the risk and prognosis of CRC,8–10 since they share several common risk factors, such as obesity, smoking, drinking, the western diet, and lack of exercise.11 T2DM may contribute to the development of CRC through several mechanisms, including hyperglycemia, oxidative stress, and chronic inflammation.9 Encouragingly, a serials of epidemiologic studies,12–14 but not all,15,16 had shown a lower risk and mortality of CRC associated with metformin use. Several basic researches also demonstrated that metformin inhibited cancer cell proliferation, metabolism, and angiogenesis through activation of adenosine monophosphate-activated protein kinase (AMPK) and inhibition of mammalian target of rapamycin (mTOR) signaling pathway.17–19 Metformin may have multiple activities against tumor, which represent a promising perspective in cancer therapy.20 To date, though the antineoplastic effects of metformin are biologically plausible, existing data remain controversial. For example, several studies15,16 have shown that metformin does not reduce the incidence of CRC in patients with T2DM.
Considering these controversial contexts, we performed a meta-analysis based on existing observational studies and randomized controlled trials (RCTs) to determine whether use of metformin may protect T2DM patients against CRC. Since high prevalence and poor prognosis of CRC, a potential antitumor role of metformin would markedly impact on clinical and public health.
METHODS
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines.21
Search Strategy
We (HXK and STT) independently searched Medline, Web of Science, and the Cochrane Library databases for all relevant studies before August 2015. Medical subject heading (Mesh) terms and keywords were used in the search included “metformin,” “biguanide,” “colon neoplasm,” and “colorectal cancer.” Two authors reviewed the titles and abstracts of studies identified in the search independently in order to exclude unrelated studies. We examined the remaining full articles and references to determine whether it contained any additional papers.
Eligibility Criteria
Eligible articles were considered in this meta-analysis if they met the following criteria: original articles reported estimated risks with 95% confidence interval (CI); evaluated association between CRC and metformin use; T2DM was identified before CRC diagnosis based on medical or pathological diagnosis; studies published in English were included. When there were multiple publications from the same cohort, we extracted information from the most recent comprehensive study.
Data Extraction and Quality Assessment
Two researchers (HXK and STT) extracted data from included studies independently by scrutinizing the full text. The following information were collected from eligible articles: authors, year, design, location, time period, exposure ascertainment, outcome assessment, total subjects, colon cancer cases and confounding variables adjusted, and so on. In order to better understand the risk of bias among included studies, the Newcastle-Ottawa Scale22 was applied for quality assessment in observational studies. The Cochrane Collaboration's tool was also used to assess the risk of bias in RCTs. All methodological quality of eligible studies were performed by 2 authors independently (HXK and STT). Any discrepancies were resolved by discussions or with the third researcher (SLM).
Statistical Analysis
Pooled ORs (HRs) and 95% CI were calculated using a random-effects model23 if the heterogeneity was considerable, and a fixed-effects model was performed otherwise. Adjusted estimates reported in studies were used for meta-analysis in order to account for confounding factors. We assessed heterogeneity among individual studies by 2 methods: Cochran Q test and I2.24 Statistically significant for heterogeneity was considered if P ≤ 0.05 and/or I2 > 30%. In order to investigate sources of heterogeneity, we performed subgroup analyses25 by grouping study location, design, adjusted for other antidiabetic medications (ADMs). Besides we conducted sensitivity analyses by excluding 1 study each time and rerunning the analysis to verify the robustness of the overall results. Publication bias was assessed by conducting statistical tests for funnel plot asymmetry as well as Egger test and Begg test. A probability level <0.05 was considered statistically significant and all P values were 2 tailed. All statistical analyses were conducted using Stata software (version 11.0; StataCorp, College Station, TX).
RESULTS
There were 1330 studies that were identified by the search strategy. Among them, only 20 observation studies and one RCT were finally included in this meta-analysis (Figure 1). These studies cumulatively included 16,786 cases of CRC in 1,086,268 patients with T2DM. There were three Taiwanese studies26–28 from the same cohort, therefore, only one27 of them was included in the analysis for metformin and CRC incidence. Likewise, four United Kingdom (UK) studies15,29–31 from the same cohort and only one30 of them was included.
FIGURE 1.
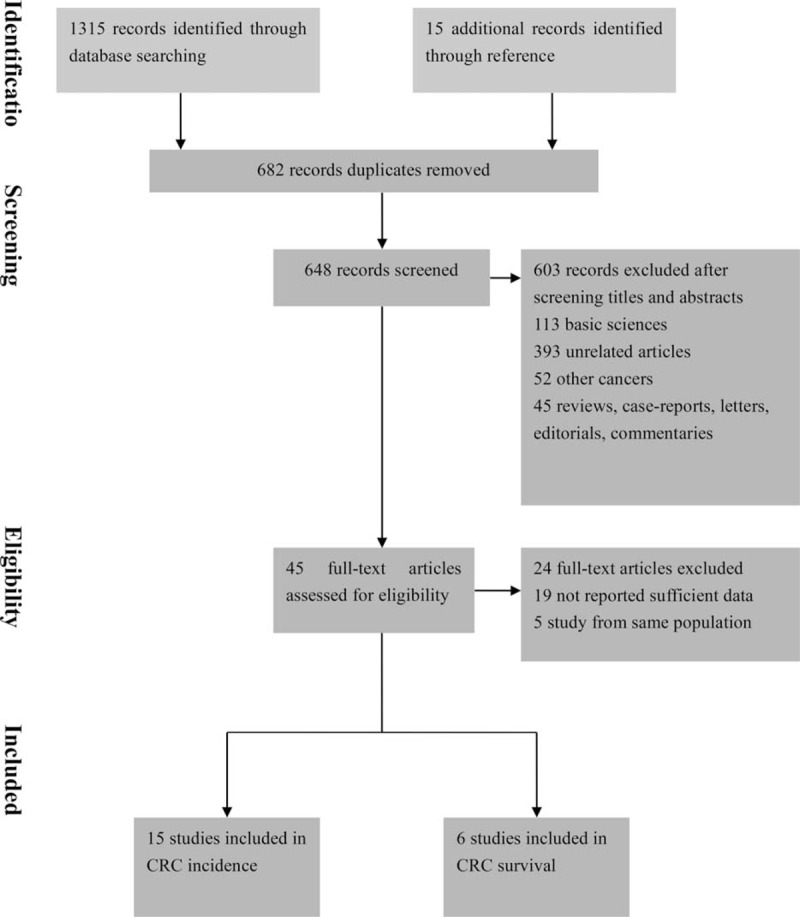
Flow diagram summarizing study identification and selection.
Characteristics and Quality of Included Studies
The characteristics of included studies are shown in Tables 1 and 2. Fifteen studies of them evaluated the association between CRC incidence and metformin, while other 6 studies assessed survival benefits associated with metformin exposure. Seventeen studies were from the Western population (7 based in the United States (US), 10 based in Europe), 3 studies were performed in the Asian population, and 1 was a multicenter RCT across the US, Europe, and Asia. Seventeen selected studies were published in recent 5 years (2010–2015). Two articles16,32 reported 4 different cohorts, while an article33 reported colon and rectal cancer, respectively. In most studies, exposure was ascertained from pharmacy database and outcome assessment was based on standard diagnostic codes. Nonresponse rate in case–control studies and duration of follow-up in cohort were inconsistently reported.
TABLE 1.
Characteristics of Included Studies Assessing the Risk of Colorectal Cancer in Patients With Diabetes Mellitus Treated With Metformin
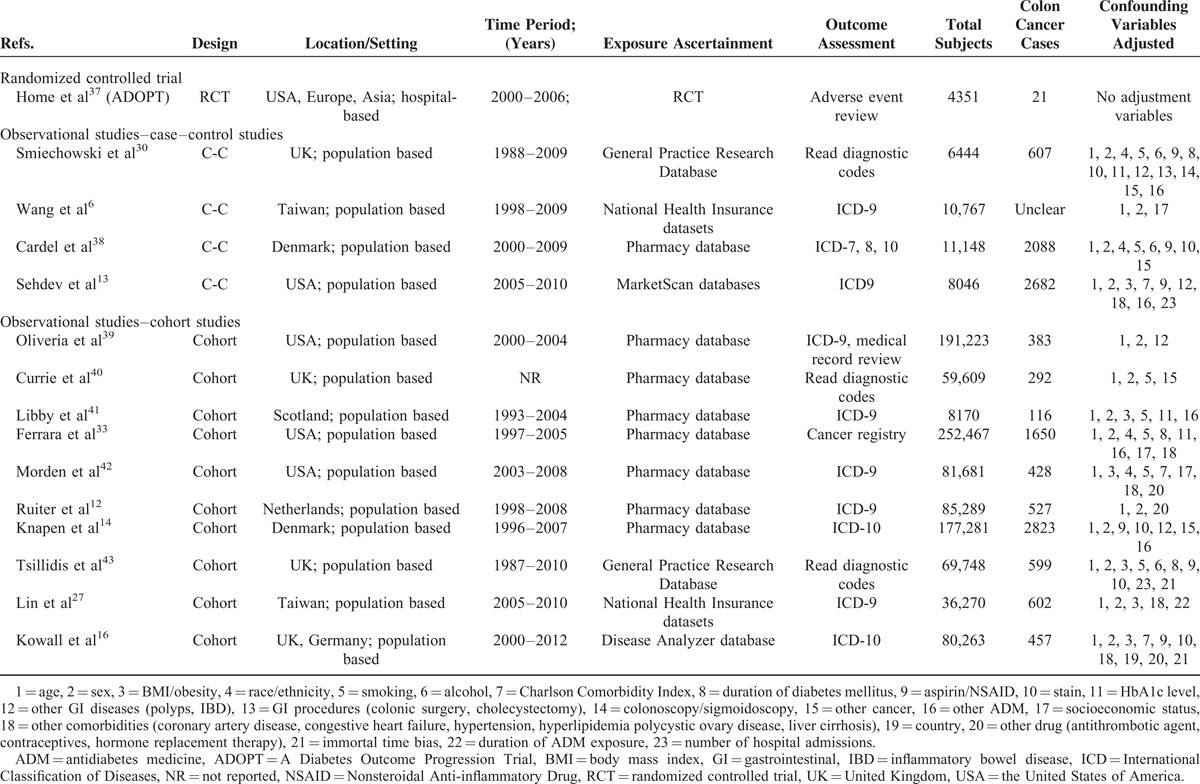
TABLE 2.
Characteristics of Included Studies Assessing the Prognosis of Colorectal Cancer in Patients With Diabetes Mellitus Treated With Metformin
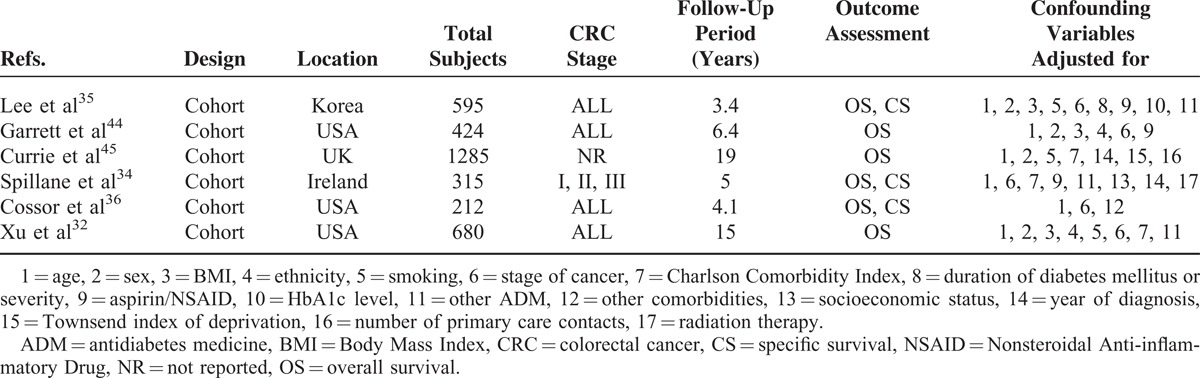
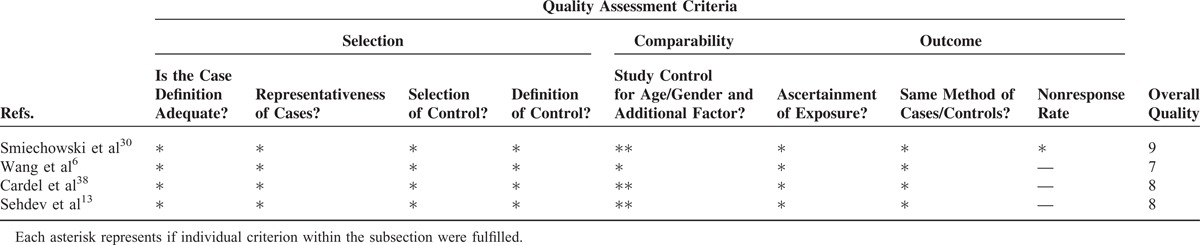
The overall methodological quality was moderate to high (Tables 3–5). Using the Newcastle-Ottawa scale quality tool, the quality of included studies was moderate or high. The quality of the randomized trials was moderate according to the Cochrane Collaboration's tool.
TABLE 3.
Newcastle-Ottawa Scale for Assessment of Quality of in Included Cohort Studies
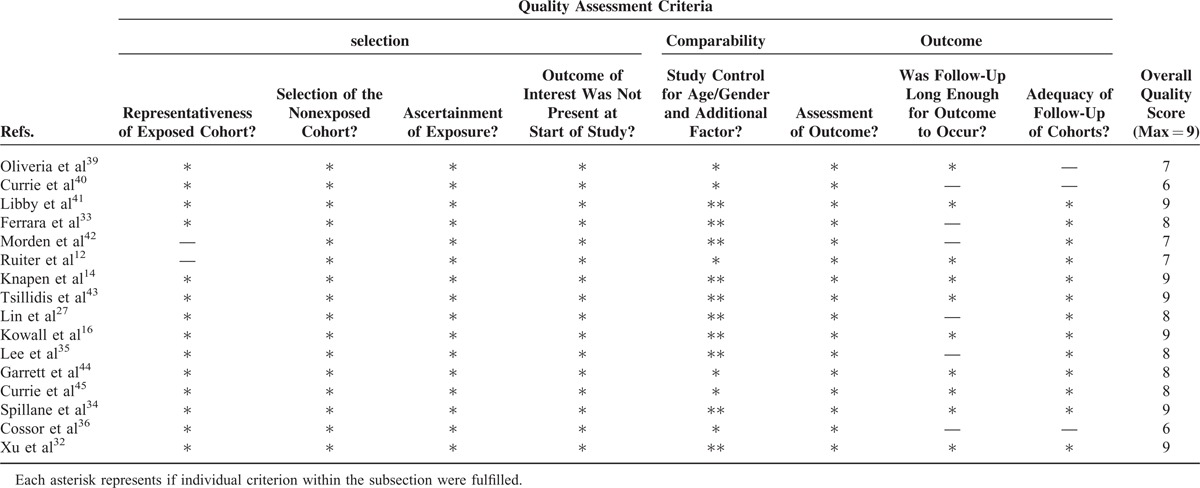
TABLE 5.
Cochrane Collaboration's Tool for Assessment of Quality of Randomized Controlled Trials

TABLE 4.
Newcastle-Ottawa Scale for Assessment of Quality of Included Case–Control Studies
Metformin Exposure and Risk of CRC
Among the 14 observational studies that reported CRC incidence, 5 demonstrated an apparent protective association and the other 9 studies showed no statistically significant relationship. The pooled analyses of observational studies demonstrated that the use of metformin was associated with a statistically significant 10% reduction in CRC incidence among T2DM patients (OR = 0.90, 95% CI: 0.85–0.96) (Figure 2), which was consistent with previous meta-analysis. The results of subgroup analyses for the association between metformin use and CRC risk are demonstrated in Table 6. Importantly, we performed sensitivity analyses by excluding 1 article each time and recalculated the pooled OR for remaining studies. Results demonstrated overall pooled estimates were robust and the chemopreventive effect of metformin persisted in CRC patients with T2DM. There was considerable heterogeneity among studies (I2 = 46.5%, P = 0.02), which could be partly due to study design. There was no evidence of publication bias in our analysis, based on the Egger test (P = 0.27) or Begg test (P = 0.14), and on visual inspection of the funnel plot (Figure 3).
FIGURE 2.
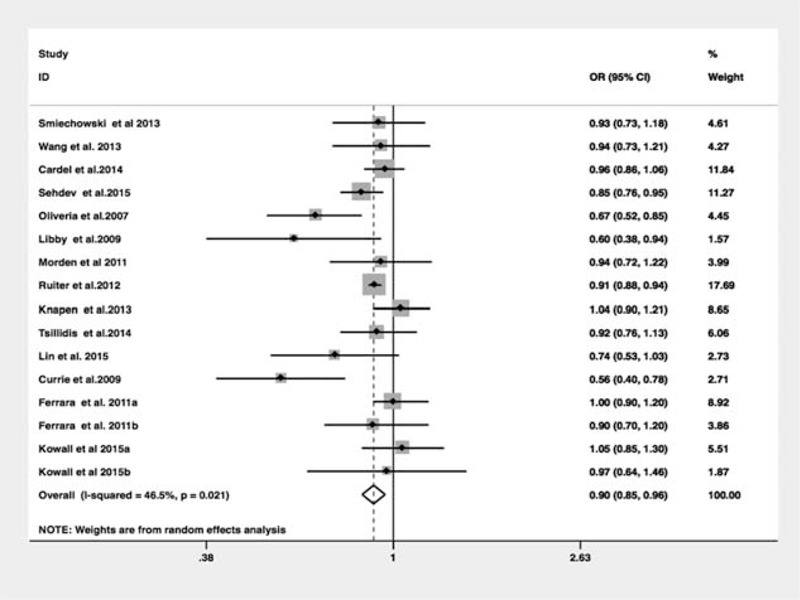
Meta-analysis of the association between metformin use and colorectal cancer risk in patients with diabetes mellitus.
TABLE 6.
Subgroup Analysis of Studies Comparing the Association Between CRC Risk and Metformin
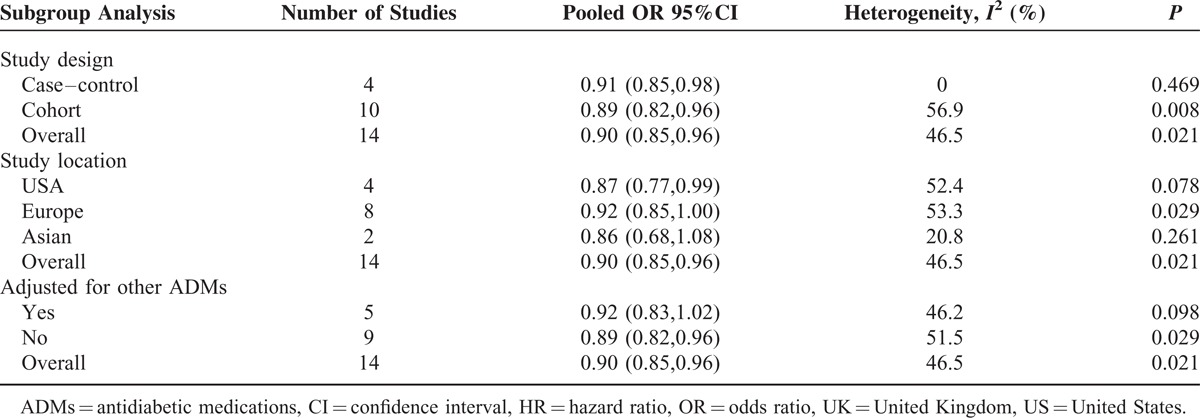
FIGURE 3.
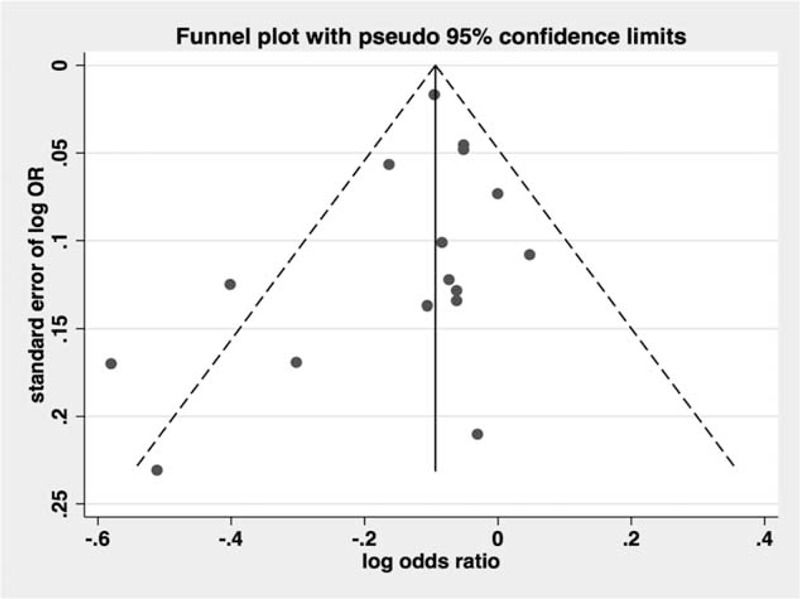
Funnel plot analysis to detect publication bias.
Metformin and Morality of CRC
Among the six selected studies, all reported overall survival (OS) and three34–36 also presented CRC-specific survival (CS). The pooled HR of OS was 0.68 (95% CI: 0.58–081) (Figure 4), with some evidence of heterogeneity (I2 = 62.4%, P = 0.01). The pooled HR of CS was 0.66 (95% CI: 0.50–0.87), with no evidence of heterogeneity (I2 = 0%, P = 0.88). Our study showed that metformin use in CRC patients with T2DM moderately reduced both all-cause death and CRC-specific mortality. Subgroup and sensitivity analyses were not performed since the number of included studies was limited. Substantial heterogeneity was present among OS and no heterogeneity existed for CS. Because of the limited number of included studies, it was difficult to confirm whether the publication bias exists in our meta-analysis.46
FIGURE 4.
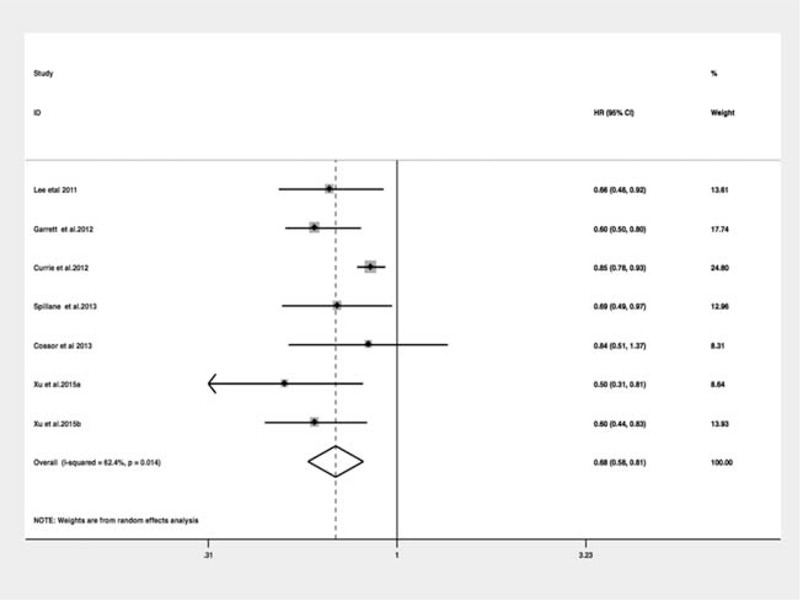
Meta-analysis of the association between metformin use and all-cause mortality in patients with diabetes mellitus.
DISCUSSION
Based on 20 observational studies and 1 RCT, our meta-analysis showed metformin was associated with a slight, yet statistically significant, protective effect (10% risk reduction) on CRC risk among patients with T2DM. The benefits associated with metformin were stable even after sensitivity analyses. It was also in line with results of previous meta-analysis.47,48 Though identified in observational studies, the potential antineoplastic effect of metformin is unproven in the RCT (OR = 0.69, 95% CI: 0.26–1.82). This may be due to the fact that the trial was not primarily designed to explore the effect of metformin on CRC risk, which inevitably introduced some bias into the trial. Besides, 1 RCT might have no enough power to detect a significant association between metformin with CRC risk. Therefore, further specially designed RCTs are needed to confirm this protective effect.
Notably current studies indicated that the magnitude of chemopreventive effect was not as obvious as previous studies.48 Recently, more researchers were aware of shortcoming and potential bias of observational studies.49,50 In order to avoid overestimation the effect of metformin, authors minimized time-related bias49,50 and adjusted more confounding factors as far as possible. Subgroup analyses also suggested that the protective association between metformin and CRC risk was not different among different regions (US OR = 0.87, 95% CI: 0.77–0.99; Europe OR = 0.92, 95% CI: 0.85–1.00; Asian OR = 0.86, 95% CI: 0.68–1.08). More importantly, survival advantages were observed among CRC patients with T2DM in our analysis. Patients taking metformin had a better prognosis compared with nonusers, which achieved estimated OS benefits of 32%.
Although previous studies indicated that metformin was associated with a reduction in CRC risk, potential biologic mechanisms underlying the antitumor effect of metformin was still pending. There is a growing body of evidence indicating that metformin exerts the anticancer activity through its systemic effects as well as cellular effects. The systemic effects of metformin can potentially counteract the Warburg effect by reducing hyperglycemia.51 Warburg effect is a crucial metabolic feature in cancer cells that facilitates bypass senescence.52 The cellular effects are associated with activation of AMPK and consequently inhibition of mTOR pathway,17,53 which plays a critical role in cell proliferation and carcinogenesis among many tumors. Activation of mTOR closely correlates with cancer progression, resistance to chemotherapy, and poor prognosis.54 Furthermore, metformin may also promote tumor cell senescence through suppressing cyclin D1 expression.55 The antitumor effects have also been illustrated in animal models of CRC. Tomimoto et al18 reported that metformin could suppress intestinal polyposis in the adenomatous polyposis coli (APCMin/+) mice. Besides, metformin could also inhibit the formation of colorectal aberrant crypt foci in the murine model of azoxymethane-induced colitis-associated cancer.56 These evidence from in vivo and in vitro strengthen the role of metformin as one of the promising candidates for cancer therapeutics.
The strength of our systematic analysis consists in including comprehensive studies, large numbers of patients, as well as assessment of the survival benefits between metformin and CRC. Zhang et al48 firstly performed a meta-analysis of metformin and CRC risk in 2011, however, they included only 4 studies and did not perform subgroup or sensitivity analysis since limited numbers. Recently, Singh et al47 performed a meta-analysis of ADMs and CRC risk. They included 10 articles and failed to evaluate specifically metformin and CRC. Both studies did not assess the effect of metformin on CRC survival. In fact, both of them showed a chemopreventive effect of metformin, though variable in magnitude. The magnitude of protective effect in our study was less evident comparing with the meta-analysis in 2011 and similar with that of Singh's analysis. This may be due to the larger numbers of the included studies. Meanwhile, we speculated that recently published studies avoided time-related bias and took a wide range of confounding variables into consideration. The conclusion of our study about metformin affection on CRC risk might be more scientific and credible. CRC survival benefits associated with metformin in our results also supported the antitumor effect of metformin. Of note, adjusted estimates were used to calculate the summary results instead of unadjusted ones in order to avoid potential confounding factors. Besides we performed subgroup and sensibility analyses to ensure stability of the association and identify factors responsible for heterogeneity.
However, our study also has several limitations that merit further consideration. Firstly, our pooled results were based on data from observational studies, while only 1 RCT is feasible. Observational studies had methodical shortcomings and are prone to time-related biases, such as immortal time bias and time-lagging issues.49 This may potentially overestimate the apparently protective effect of metformin. Secondly, the adjusted potential covariates of included studies were incomplete and inconsistent. Moreover, some confounding factors such as dietary consumptions, physical activity, and screening colonoscopy were not well adjusted for included studies. Thirdly, the included studies were limited in reporting dose and duration of metformin use among CRC patients with T2DM. Hence, neither dose–response or duration–response association between metformin use and risk of CRC could be established. Finally, this meta-analysis was restricted to English language studies, which might introduce publication bias.
In summary, our meta-analysis demonstrated metformin use might be associated with a lower risk and better prognosis of CRC in diabetic patients based on current evidence. These data highlight the role of metformin as a potential candidate for chemopreventive drugs on CRC patients with T2DM. However, further investigations, especially well-designed RCTs, are expected to substantiate these benefits from early observational studies.
Acknowledgment
We thank Peiwei Li for assistance with data analysis.
Footnotes
Abbreviations: ACS = American Cancer Society, ADMs = antidiabetic medications, AMPK = adenosine monophosphate-activated protein kinase, APC = adenomatous polyposis coli, CI = confidence interval, CRC = colorectal cancer, CS = CRC-specific survival, HR = hazard ratio, mTOR = mammalian target of rapamycin, OR = odds ratio, OS = overall survival, RCT = randomized controlled trial, T2DM = type 2 diabetes mellitus, UK = United Kingdom, US = United States.
This work was funded by the Zhejiang provincial medical platform 2015 specialists class B (2015 RCB016), Zhejiang province key science and technology innovation team (2013TD13); the National Natural Science Foundation of China (81372623, 81302070).
Ethics statement All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
Authors’ contributions: SLM and SJM contributed to conception and design of the study. HXK and STT contributed to the data acquisition, analysis, and interpretation of the data. HXK and SLM contributed to writing and editing the manuscript. All authors commented on drafts of the paper and have approved the final draft of the manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014; 64:252–271. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010; 116:544–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.La Vecchia C, Bosetti C, Lucchini F, et al. Cancer mortality in Europe, 2000–2004, and an overview of trends since 1975. Ann Oncol 2010; 21:1323–1360. [DOI] [PubMed] [Google Scholar]
- 4.Hyodo I, Suzuki H, Takahashi K, et al. Present status and perspectives of colorectal cancer in Asia: Colorectal Cancer Working Group report in 30th Asia-Pacific Cancer Conference. Jpn J Clin Oncol 2010; 40 (Suppl. 1):i38–i43. [DOI] [PubMed] [Google Scholar]
- 5.Patel JD, Chang KJ. The role of virtual colonoscopy in colorectal screening. Clin Imaging 2015; PMID: 26298421. [DOI] [PubMed] [Google Scholar]
- 6.Wang SY, Chuang CS, Muo CH, et al. Metformin and the incidence of cancer in patients with diabetes: a nested case-control study. Diabetes Care 2013; 36:e155–e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010; 3:1451–1461. [DOI] [PubMed] [Google Scholar]
- 8.Jin T. Why diabetes patients are more prone to the development of colon cancer? Med Hypotheses 2008; 71:241–244. [DOI] [PubMed] [Google Scholar]
- 9.Zelenko Z, Gallagher EJ. Diabetes and cancer. Endocrinol Metab Clin North Am 2014; 43:167–185. [DOI] [PubMed] [Google Scholar]
- 10.Meyerhardt JA, Catalano PJ, Haller DG, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol 2003; 21:433–440. [DOI] [PubMed] [Google Scholar]
- 11.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and Cancer: a consensus report. Diabetes Care 2010; 33:1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiter R, Visser LE, van Herk-Sukel MP, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care 2012; 35:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sehdev A, Shih YC, Vekhter B, et al. Metformin for primary colorectal cancer prevention in patients with diabetes: a case-control study in a US population. Cancer 2015; 121:1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knapen LM, Dittrich ST, de Vries F, et al. Use of biguanides and the risk of colorectal cancer: a register-based cohort study. Curr Drug Saf 2013; 8:349–356. [DOI] [PubMed] [Google Scholar]
- 15.Bodmer M, Becker C, Meier C, et al. Use of metformin is not associated with a decreased risk of colorectal cancer: a case-control analysis. Cancer Epidemiol Biomarkers Prev 2012; 21:280–286. [DOI] [PubMed] [Google Scholar]
- 16.Kowall B, Stang A, Rathmann W, et al. No reduced risk of overall, colorectal, lung, breast, and prostate cancer with metformin therapy in diabetic patients: database analyses from Germany and the UK. Pharmacoepidemiol Drug Saf 2015. [DOI] [PubMed] [Google Scholar]
- 17.Algire C, Amrein L, Zakikhani M, et al. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr Relat Cancer 2010; 17:351–360. [DOI] [PubMed] [Google Scholar]
- 18.Tomimoto A, Endo H, Sugiyama M, et al. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci 2008; 99:2136–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001; 108:1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li D. Metformin as an antitumor agent in cancer prevention and treatment. J Diabetes 2011; 3:320–327. [DOI] [PubMed] [Google Scholar]
- 21.Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for a systematic review and meta-analysis of individual participant data The PRISMA-IPD Statement. JAMA 2015; 313:1657–1665. [DOI] [PubMed] [Google Scholar]
- 22.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [cited 5 May 2012]. 2012; http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 25.Thompson SG. Why sources of heterogeneity in meta-analysis should be investigated. BMJ 1994; 309:1351–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng CH. Diabetes, metformin use, and colon cancer: a population-based cohort study in Taiwan. Eur J Endocrinol 2012; 167:409–416. [DOI] [PubMed] [Google Scholar]
- 27.Lin CM, Huang HL, Chu FY, et al. Association between gastroenterological malignancy and diabetes mellitus and anti-diabetic therapy: a nationwide, population-based cohort study. PLoS ONE 2015; 10:e0125421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee MS, Hsu CC, Wahlqvist ML, et al. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer 2011; 11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology 2004; 127:1044–1050. [DOI] [PubMed] [Google Scholar]
- 30.Smiechowski B, Azoulay L, Yin H, et al. The use of metformin and colorectal cancer incidence in patients with type II diabetes mellitus. Cancer Epidemiol Biomarkers Prev 2013; 22:1877–1883. [DOI] [PubMed] [Google Scholar]
- 31.Qiu H, Rhoads GG, Berlin JA, et al. Initial metformin or sulphonylurea exposure and cancer occurrence among patients with type 2 diabetes mellitus. Diabetes Obes Metab 2013; 15:349–357. [DOI] [PubMed] [Google Scholar]
- 32.Xu H, Aldrich MC, Chen Q, et al. Validating drug repurposing signals using electronic health records: a case study of metformin associated with reduced cancer mortality. J Am Med Inform Assoc 2015; 22:179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrara A, Lewis JD, Quesenberry CP, Jr, et al. Cohort study of pioglitazone and cancer incidence in patients with diabetes. Diabetes Care 2011; 34:923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spillane S, Bennett K, Sharp L, et al. A cohort study of metformin exposure and survival in patients with stage I–III colorectal cancer. Cancer Epidemiol Biomarkers Prev 2013; 22:1364–1373. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Kim TI, Jeon SM, et al. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int J Cancer 2012; 131:752–759. [DOI] [PubMed] [Google Scholar]
- 36.Cossor FI, Adams-Campbell LL, Chlebowski RT, et al. Diabetes, metformin use, and colorectal cancer survival in postmenopausal women. Cancer Epidemiol 2013; 37:742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Home PD, et al. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 2010; 53:1838–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cardel M, Jensen SM, Pottegard A, et al. Long-term use of metformin and colorectal cancer risk in type II diabetics: a population-based case-control study. Cancer Med 2014; 3:1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliveria SA, Koro CE, Ulcickas Yood M, et al. Cancer incidence among patients treated with antidiabetic pharmacotherapy. Diabetes & Metabolic Syndrome. Clinical Research & Reviews 2008; 2:47–57. [Google Scholar]
- 40.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009; 52:1766–1777. [DOI] [PubMed] [Google Scholar]
- 41.Libby G, et al. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009; 32:1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morden NE, et al. Further exploration of the relationship between insulin glargine and incident cancer: a retrospective cohort study of older Medicare patients. Diabetes Care 2011; 34:1965–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsillidis KK, et al. Metformin does not affect cancer risk: a cohort study in the U.K. Clinical Practice Research Datalink analyzed like an intention-to-treat trial. Diabetes Care 2014; 37:2522–2532. [DOI] [PubMed] [Google Scholar]
- 44.Garrett CR, et al. Survival advantage observed with the use of metformin in patients with type II diabetes and colorectal cancer. Br J Cancer 2012; 106:1374–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Currie CJ, et al. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 2012; 35:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343:d4002. [DOI] [PubMed] [Google Scholar]
- 47.Singh S, Singh H, Singh PP, et al. Antidiabetic medications and the risk of colorectal cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2013; 22:2258–2268. [DOI] [PubMed] [Google Scholar]
- 48.Zhang ZJ, Zheng ZJ, Kan H, et al. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: a meta-analysis. Diabetes Care 2011; 34:2323–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 2012; 35:2665–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gandini S, Puntoni M, Heckman-Stoddard BM, et al. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila) 2014; 7:867–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Del Barco S, Vazquez-Martin A, Cufi S, et al. Metformin: multi-faceted protection against cancer. Oncotarget 2011; 2:896–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kondoh H, Lleonart ME, Gil J, et al. Glycolytic enzymes can modulate cellular life span. Cancer Res 2005; 65:177–185. [PubMed] [Google Scholar]
- 53.Klumpen HJ, Beijnen JH, Gurney H, et al. Inhibitors of mTOR. Oncologist 2010; 15:1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woodard J, Joshi S, Viollet B, et al. AMPK as a therapeutic target in renal cell carcinoma. Cancer Biol Ther 2010; 10:1168–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ben Sahra I, Laurent K, Loubat A, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 2008; 27:3576–3586. [DOI] [PubMed] [Google Scholar]
- 56.Hosono K, Endo H, Takahashi H, et al. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Mol Carcinog 2010; 49:662–671. [DOI] [PubMed] [Google Scholar]


