Abstract
The incidence of cutaneous and subcutaneous metastases from atypical laryngeal carcinoids is approximately 20%. However, the pathogenesis and natural history of, and prognostic factors for, the condition remain poorly understood. We reported a 54-year-old female presented with cutaneous and subcutaneous metastases from atypical laryngeal carcinoid. Laryngoscopy revealed a 0.5 × 1.5-cm reddish mass on the laryngeal surface of the epiglottis. Under general anesthesia, a biopsy sample was obtained via suspension laryngoscopy. Routine pathology revealed atypical laryngeal carcinoid. Immunohistochemical staining of the sections of primary tumor was positive for cytokeratin, chromogranin A, synaptophysin, hypoxia-inducible factor-1α, P53, and CD56. GLUT-1, p-Akt, and PI3K were negative. The Ki-67 index was 15%. Supraglottic laryngectomy and selective right-neck dissection were performed. After 6 months, the patient complained of pain in the right wall of the chest; multiple cutaneous and subcutaneous nodules were evident at that site and in the abdomen. An abdominal nodule was biopsied and pathology revealed that the atypical metastatic carcinoid had metastasized to both cutaneous and subcutaneous areas of the abdomen. Chemotherapy was then prescribed. Currently, the intrathecal drug delivery system remains in place. No local recurrence has been detected. Furthermore, we systematically reviewed clinical manifestations of the disease, pathogenesis, prognostic factors, and treatment. The metastasis rate (cutaneous and subcutaneous) was approximately 12.2%. Thirty patients (62.5%) with cutaneous and subcutaneous metastases exhibited contemporaneous lymph node invasion. The 3-, 5-, and 10-year survival rates were 44.0%, 22.0%, and 13.0%, respectively.
The prognosis of patients with atypical laryngeal carcinoids was poor. Relevant prognostic factors included the level of p53, human papilloma virus status, certain hypoxic markers, and distant metastasis. No optimal treatment for such metastases has yet been defined.
INTRODUCTION
Laryngeal neuroendocrine carcinomas (NECs) are rare, constituting <1% of all tumors of the larynx. Four histological subtypes are distinguished based on the extent of differentiation and cell size. Well and moderately differentiated NECs are termed typical and atypical carcinoids, respectively. Poorly differentiated NCLs are divided into small- and large-cell NECs.1,2 The most frequent laryngeal NEC is the atypical carcinoid, followed by small-cell NEC, carcinoid tumor, and large-cell NEC.3
The prognosis of laryngeal NEC varies by histopathological type.2 A meta-analysis of 436 patients with laryngeal NECs found that the 5-year disease-specific survival was 100% for patients with typical carcinoids, 53% for those with atypical carcinoids, and 19% for those with small-cell carcinomas. Prognostic factors included distant metastasis. An atypical carcinoid of the larynx is a more aggressive type of NEC, often associated with multiple distant metastases.4,5 Metastatic sites include the cervical and distant lymph nodes, lung, bones, skin, subcutaneous tissues, mediastinum, liver, heart, pancreas, diaphragm, peritoneum, gastrointestinal tract, prostate, breast, brain, dura mater, pleura, testicles, and muscles.4 Lymph node metastases are the most common (40%), followed by skin and subcutaneous metastases (20%), and metastases at other sites (40%).6–8 The prognoses of patients with atypical laryngeal carcinoids are relatively poor; the 5-year survival rate is approximately 50%. Death is usually caused by metastatic disease rather than local recurrence.4 Although the incidence of cutaneous and subcutaneous metastases from atypical laryngeal carcinoids is approximately 20%, few systematic analyses of clinical manifestations or treatment of such metastases have been reported. The precise means by which distant metastasis and local recurrence develop remain unclear, as do relevant prognostic factors.
To date, the only effective treatment appears to be surgery. The disease is refractory to chemotherapy, and any role for radiotherapy is controversial.9 Thus, new treatments are required to improve long-term survival. Targeted therapies have recently been used to treat other cancers,10,11 including NECs of other sites. Targets include vascular endothelial growth factor, platelet-derived growth factor, and the mammalian target of rapamycin; such treatments have improved the progression-free survival times of patients with pancreatic NEC, pulmonary large-cell-type NEC, and prostate NEC.12–14 However, no report on targeted therapy of laryngeal NEC has yet been described.
Our previous study15 and additional work16 have shown that positron emission tomography/computed tomography (PET/CT) detected high-level uptake of [18F]-fluoro-2-deoxy-d-glucose (FDG) by laryngeal NECs, as is also true of other head-and-neck cancers.17–20 Many studies have found that FDG uptake is associated with overexpression of glucose transporter-1 (GLUT-1),20–23 which is associated with metastasis and poor prognosis of many human cancers.22,24,25 NECs also express high levels of GLUT-126–28 with certain biological consequences.26 We previously found that targeted inhibition of GLUT-1 decreased glucose uptake by, and inhibited proliferation of, Hep-2 cells,29 and enhanced the radiosensitivity of laryngeal carcinoma Hep-2 cells.30 Thus, we proposed that GLUT-1 targeting may be useful for the treatment of laryngeal NECs.
Here, we report a patient exhibiting cutaneous and subcutaneous metastases from an atypical laryngeal carcinoid and review clinical manifestations, possible pathogenesis, prognostic factors, and treatments. We measured the levels of GLUT-1 mRNA and protein, and screened for human papilloma virus (HPV), cytomegalovirus (CMV), and Epstein–Barr virus (EBV).
CASE REPORT
Presenting Concerns
A 54-year-old female presented with a sore throat and radiating pain in the right ear lasting over 1 year in duration. She denied hoarseness, respiratory distress, dysphagia, and fever. Her past medical history included hypertension during the 3 years prior, blood pressure was controlled by oral irbesartan (10 mg, per day).
Clinical Findings
Laryngoscopy revealed a 0.5 × 1.5-cm reddish mass on the laryngeal surface of the epiglottis (Figure 1). Movements of both vocal cords were normal. Magnetic resonance imaging (MRI) revealed a 0.8 × 1.3-cm lesion in the epiglottis. T1-weighted imaging was isointense; T2-weighted imaging hyperintense; and diffusion-weighted imaging of high signal intensity. The lesion underwent heterogeneous enhancement following administration of gadolinium-DTPA (Figure 2). However, the preoperative PET/CT was not done.
FIGURE 1.
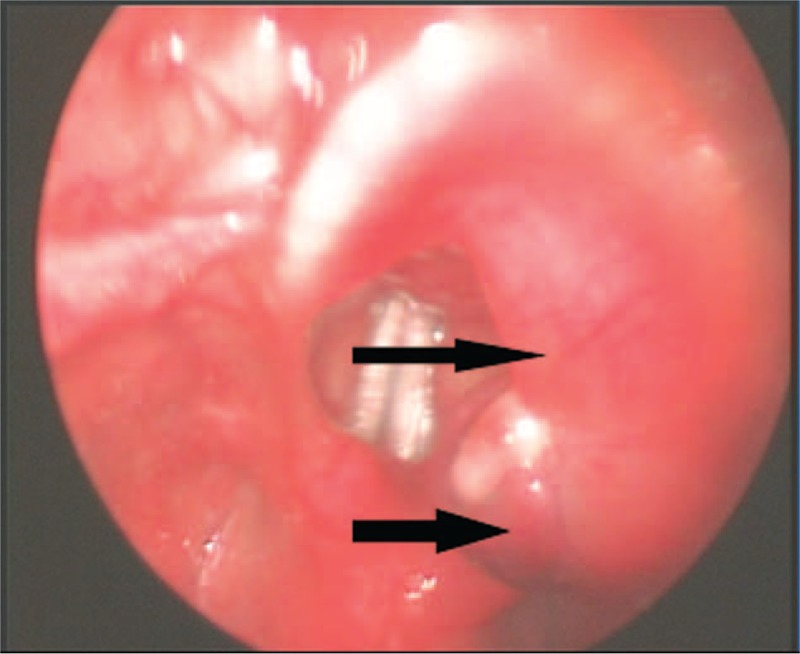
Laryngoscopy revealed a 0.5 × 1.5-cm reddish mass on the laryngeal surface of the epiglottis.
FIGURE 2.
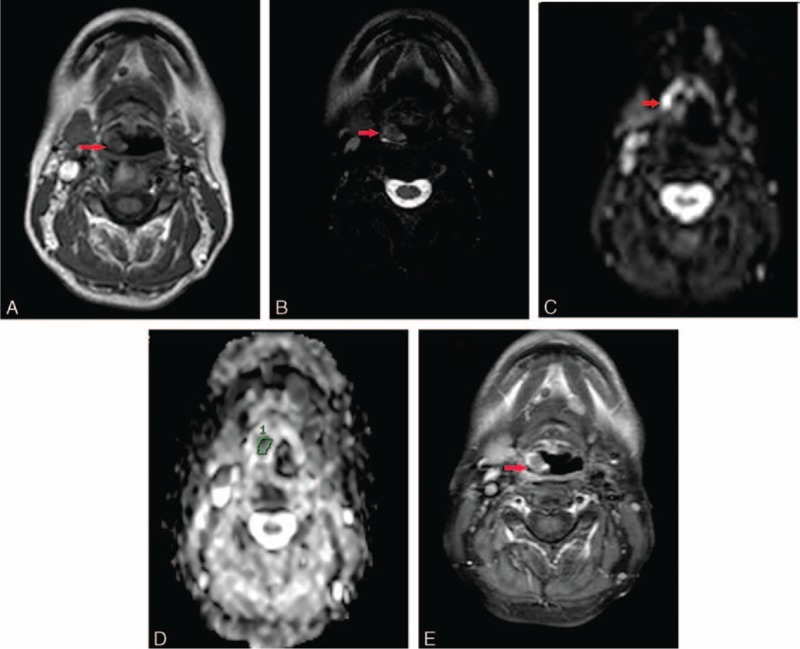
MRI revealed a 0.8 × 1.3-cm lesion in the epiglottis. T1-weighted imaging was isointense (A), but T2-weighted imaging was hyperintense (B). diffusion-weighted imaging exhibited high signal intensity (C). The apparant diffusion coefficient value was 0.741 × 10−3 mm2/s (D). The lesion exhibited heterogeneous enhancement (E). MRI = magnetic resonance imaging.
The institutional review board (IRB no.2015427) of The First Affiliated Hospital, College of Medicine, Zhejiang University (Hangzhou City, China) approved the present study. Written informed consent was obtained from the patient before inclusion.
Therapeutic Focus and Assessment
The process of the patient received was listed in Table 1. Under general anesthesia, a biopsy sample was obtained via suspension laryngoscopy. Analysis of the frozen section suggested that only a small-cell tumor was present; therefore, it was necessary to immunohistochemically differentiate a myoepithelial tumor from an atypical carcinoma. Routine pathology revealed that the tumor cells were uniform in size, exhibited marked heteromorphy, had an eosinophilic cytoplasm, and were arranged into nests and cords containing prominent blood sinuses. Immunohistochemical staining of the sections of primary tumor was positive for cytokeratin, chromogranin A, synaptophysin, hypoxia-inducible factor-1α (HIF-1α), P53, and CD56. GLUT-1, p-Akt, and PI3K were negative. The Ki-67 index was 15% (Figure 3). These histopathological features confirmed the presence of an atypical carcinoid. On May 7th 2014, supraglottic laryngectomy and selective right-neck dissection were performed under general anesthesia (Figure 4). Postoperatively, the histopathological features of paraffin sections were confirmed to be those of an atypical carcinoid; the right cervical lymph nodes and the resection margin were negative. The pathological diagnosis was pT2N0M0. The postoperative course was uneventful; no surgical morbidity was evident. Postoperative radiotherapy was not scheduled.
TABLE 1.
Timeline

FIGURE 3.
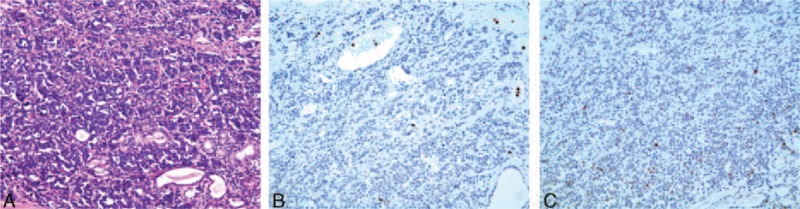
Pathology revealed that the tumor cells were uniform in size, markedly heteromorphic with eosinophilic cytoplasm, and formed nests and cords containing prominent blood sinuses (A) (HE × 200). Immunohistochemical staining was positive for hypoxia-inducible factor-1α (HIF-1α) (B) and p53 (C) (EliVision, ×200).
FIGURE 4.
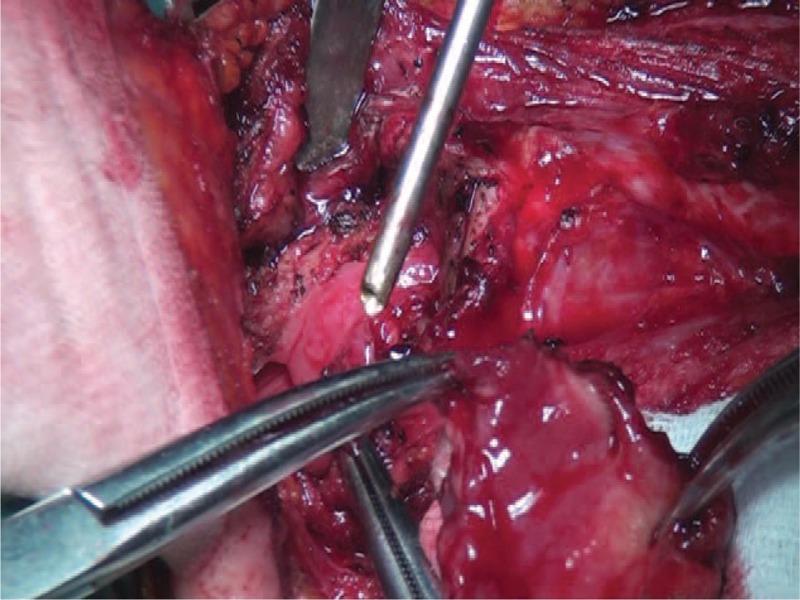
Surgical section revealed a reddish lesion in the laryngeal surface of the epiglottis.
Follow-Up and Outcomes
The patient received regular monthly follow-ups. On October 9th 2014, PET/CT revealed no recurrence or distant metastasis. In November 2014, the patient complained of pain in the right wall of the chest; multiple cutaneous and subcutaneous nodules were evident at that site and in the abdomen (Figure 5). An abdominal nodule was biopsied and pathology revealed that the atypical metastatic carcinoid had metastasized to both cutaneous and subcutaneous areas of the abdomen. Chemotherapy was then prescribed (capecitabine, 100 mg orally/day and 150 mg orally/night, days 1–14; and temozolomide, 300 mg orally/day, days 2–5). However, even after 2 cycles of chemotherapy, the nodules did not shrink in size and, indeed, several new cutaneous and subcutaneous metastases developed. Pain was not resolved by chemotherapy but was relieved with oral oxycontin. Another chemotherapy regime was then prescribed (octreotide, 30 mg intramuscularly, 1 dose). However, this did not control the pain (scored using a visual analog scale as 6) from the multiple nodules. Pain was relieved with the aid of an intrathecal drug delivery system (ropivacaine, 4 mL + ketamine, 60 mg + morphine, 160 mg; maintenance dose, 6 mg/day). On May 20th 2015, MRI revealed a metastasis in the lumbar vertebrae. Hence, a new chemotherapeutic regime was prescribed (2 cycles: irinotecan, 350 mg/m2 by intravenous infusion); the nodules did not respond. Currently, the intrathecal drug delivery system remains in place. No local recurrence has been detected.
FIGURE 5.
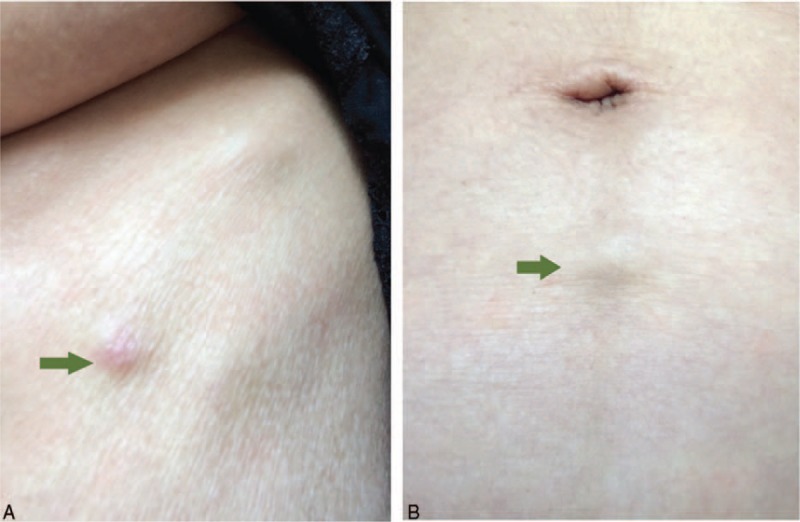
Multiple cutaneous and subcutaneous nodules were evident in the right wall of the chest (A) and the abdomen (B).
GLUT-1, HIF-1α, p-Akt, and PI3K mRNA levels of frozen tissues of primary tumor and precancerous lesion (laryngeal papilloma) as control were measured using real-time reverse transcription-polymerase chain reaction (RT-PCR). The results showed that the expression of GLUT-1, p-Akt, and PI3K mRNA was higher in the atypical carcinoid than in precancerous lesion and in the laryngeal squamous cell carcinoma (Figure 6). GLUT-1, HIF-1α, p-Akt, and PI3K protein levels were analyzed using a BAC Protein Quantification Kit and Western blot analysis. The results showed that the expression of GLUT-1, p-Akt, and PI3K proteins was higher in the atypical carcinoid and in the laryngeal squamous cell carcinoma than in precancerous lesion; however, there is no difference in expression between in the atypical carcinoid and in the laryngeal squamous cell carcinoma (Figure 7). HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 59, and 68 types), CMV, and EBV DNAs were detected by quantitative fluorescence PCR. During the detection of HPV, H2O was used as a negative control and an immunodeficiency virus containing the target gene was the positive control. During the detection of CMV, a CMV-negative sample was the negative control and HCMV AD-169 standard strain was the positive control. During the detection of EBV, saline was the negative control and clinical isolates of the EB virus were the positive control. In all viral detection assays, samples from healthy controls were also included. The results revealed that HPV, CMV, and EBV DNAs were negative. Each primer sample was run in triplicate.
FIGURE 6.
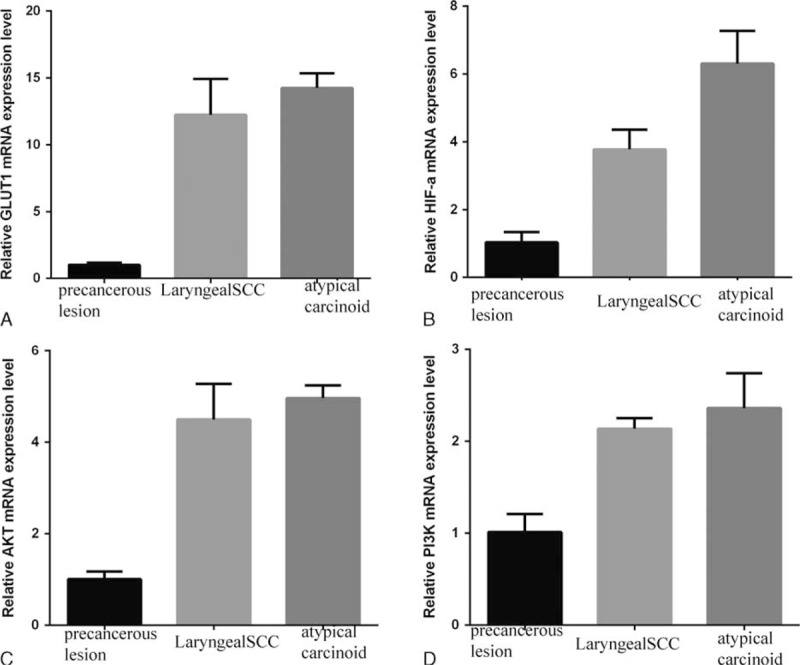
RT-PCR showed that the expression of GLUT-1 (A), HIF-1α (B), p-Akt (C), and PI3K (D) mRNA were high than these in precancerous lesion and laryngeal squamous cell carcinoma. Akt = protein kinase B, GLUT-1 = glucose transporter-1, HIF-1α = hypoxia-inducible factor-1α, PI3K = phosphatidylinositol 3-kinase, RT-PCR = reverse transcription-polymerase chain reaction.
FIGURE 7.
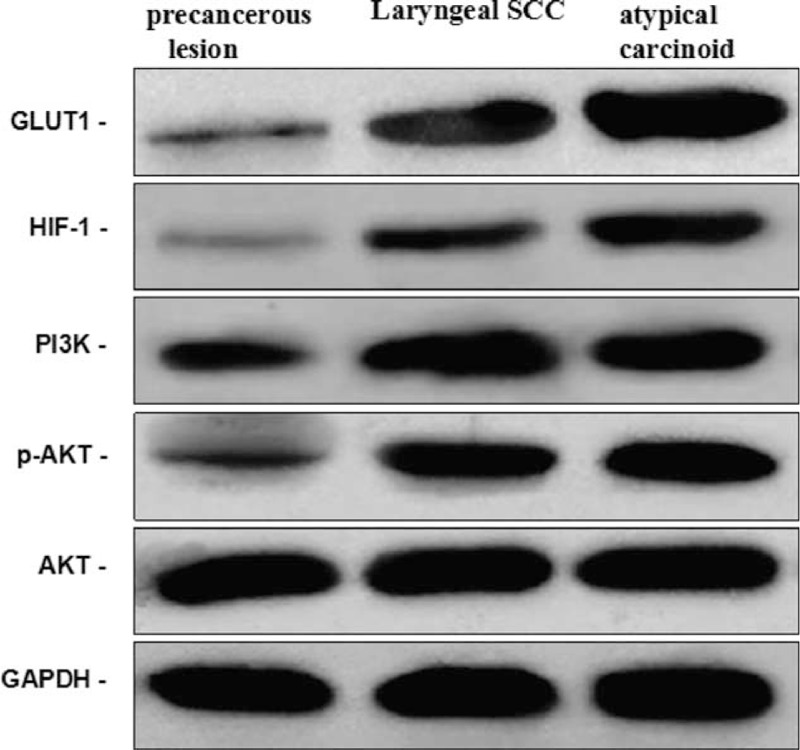
Western blot analysis showed that the expression of GLUT-1, HIF-1α, p-Akt, and PI3K proteins were high than these in precancerous lesion and laryngeal squamous cell carcinoma. Akt = protein kinase B, GLUT-1 = glucose transporter-1, HIF-1α = hypoxia-inducible factor-1α, PI3K = phosphatidylinositol 3-kinase.
DISCUSSION
An atypical laryngeal carcinoid is the most common nonsquamous cell carcinoma of the larynx. The tumor is aggressive and the prognosis relatively poor compared to a laryngeal carcinoid. However, the pathogenesis and natural history of, and prognostic factors for, the condition remain poorly understood.
Only 2 reports have explored HPV status in patients with laryngeal NECs.31,32 Giordano et al (2009)31 found no HPV DNA in a patient with a moderately differentiated laryngeal NEC (an atypical carcinoid). Halmos et al (2013)32 described 2 patients who were positive for the high-risk type of HPV (hrHPV) (1 atypical laryngeal carcinoid patient was positive for HPV 18, and 1 patient with a laryngeal small-cell NEC was positive for HPV 16). Both patients experienced relatively good outcomes compared to those of another 5 patients. It was therefore suggested that hrHPV involvement in laryngeal NECs may be associated with good prognoses. We also detected hrHPV in our current patient. We were the first to explore CMV and EBV DNA status in patients with laryngeal NECs. Such patients were negative for hrHPV, CMV, and EBV DNAs. Therefore, any relationship between laryngeal NEC and viral DNA positivity requires further investigations in larger numbers of patients.
Protein p53 is prognostic in terms of the outcomes of patients with neuroendocrine tumors.33 Giordano et al (2009)31 reported p53 overexpression in a patient with a moderately differentiated laryngeal NEC (an atypical carcinoid). Chung et al (2004)34 found that 3 of 6 patients with moderately differentiated laryngeal NECs were p53-positive. McCluggage et al (1997)35 also reported that 3 atypical laryngeal carcinoid patients were p53-positive. Overholt et al (1995)36 found that 6 of 8 patients with laryngeal neuroendocrine neoplasms (including 2 atypical carcinoids) were p53-positive. The cited authors suggested that p53 mutation may be involved in the pathogenesis of atypical carcinoids.31,34–36 Our present patient is also p53-positive.
Hypoxic markers have been explored as possible prognostic factors for NECs, including atypical carcinoids. The markers explored include GLUT-1, HIF-1α, and proteins of the associated phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathway.26–28,37–39 Ozbudak et al (2009)26 found that GLUT-1 was expressed in approximately half of all pulmonary NEC patients, and such expression was associated with an increased risk of mortality. GLUT-1 expression is regulated by HIF-1α and the PI3K/Akt signaling pathway.39 Hafner et al (2012)37 found that the PI3K/AKT pathway was activated in patients with Merkel cell carcinoma and suggested that the pathway may represent a useful new therapeutic target. Kaira et al (2013)39 reported high-level immunohistochemical expression of GLUT-1, HIF-1α, and p-Akt in 34 patients with pulmonary NECs. In addition, FDG uptake was associated with the expression of GLUT-1, HIF-1α, VEGF, and CD34. The survival of patients positive for GLUT-1 was poorer than that of others.39 In our previous study, we found that GLUT-1, HIF-1α, PI3K, and p-Akt were overexpressed in 10 patients with sinonasal and laryngeal small-cell NECs by 80%, 50%, 40%, and 40%, respectively.27 Although the expression levels of GLUT-1, HIF-1α, PI3K, and p-Akt did not correlate with survival in a small patient series, 5 patients who died (100%) were positive for GLUT-1, and 2 (40%) for HIF-1α, PI3K, and p-Akt.27 Our present patient expressed high levels of GLUT-1, HIF-1α, PI3K, and p-Akt mRNAs and proteins, as revealed by real-time RT-PCR and Western blot analysis, respectively. Thus, we propose that targeting of hypoxic markers may effectively treat laryngeal NECs.
Although the factors described above may influence pathogenesis and survival, the utilities of such molecular markers require further study. Clinicopathological factors prognostic of laryngeal NEC are well-understood; these include distant metastasis, a tumor diameter over 1 cm and tumor type.40,41 Patients with typical laryngeal carcinoids have relatively good prognoses. All atypical laryngeal carcinoids, small-cell NECs and large-cell NECs, are associated with poor outcomes.40 Of the latter 3 tumor types, atypical laryngeal carcinoid is the most common form of laryngeal NEC and exhibits a high rate (67%) of distant metastasis.42,43 Soga (2003)43 analyzed 11,842 cases with carcinoids and variant endocarinomas, and found 199 cases of atypical carcinoids in the larynx with a metastasis rate of 66.8%.43 Lymph node metastases were the most common (40%), followed by skin and subcutaneous metastases (20%) and metastases to other sites (40%).6–8
Early in 1991, Woodruff and Senie44 reported that survival was reduced when tumors triggered cutaneous and subcutaneous metastases. The cited authors reviewed 127 patients with atypical laryngeal carcinoids, 119 (94%) for whom follow-up data were available. Of these 119 patients, 22% developed skin or subcutaneous metastases.44 In 2005, Ferlito and Rinaldo45 searched the English language literature and found nearly 350 cases of atypical laryngeal carcinoids.44 As of 10 years later (in 2015), no further systematic review on this tumor type or metastases thereof has been reported. In the study described herein, we reviewed the English language literature using MEDLINE to run a PubMed/Web of Science search employing the keywords “neuroendocrine carcinoma or atypical carcinoid or moderately differentiated neuroendocrine carcinoma” with “head and neck or laryngeal or larynx” and “extrapulmonary neuroendocrine carcinoma.”
Goldman et al reported the 1st case of atypical laryngeal carcinoid in 1969. The patient also exhibited widespread subcutaneous metastases. Since 2005, an additional 42 cases (including our present case) have been described (Table 2 ).9,32,41,46–55 Of these recent cases, 23 were males and 17 females; the genders of 2 were not specified. The male-to-female ratio was thus approximately 1.4:1, lower than those reported by Woodruff and Senie (1991)44 and Ferlito et al (2005, 2006).42,45 The mean age of the 42 patients was 61 years (range, 38–85 years), and disease incidence peaked in the 5th (12 patients, 28.6%) and 6th (13 patients, 30.9%) decades of life, unlike the data reported by Woodruff and Senie.44 Of all cases, 24 (57.1%) had histories of cigarette smoking. Of all tumors, 35 (83.3%) were located in the supraglottic area, 1 (2.4%) in the glottis, 1 (2.4%) in the subglottis, and 4 at undefined laryngeal sites. The main symptoms included sore throat (45.2%), dysphagia (40.5%), and hoarseness (23.8%). Of all patients, 35 (83.3%) received surgery (11 underwent neck dissection), and 10 (28.6%) were prescribed postoperative treatments. Three (7.1%) received radiotherapy alone and 3 (7.1%) received both chemotherapy and radiotherapy. One patient (2.4%) received chemotherapy only.
TABLE 2.
Data on 42 New Cases Described in the English Language Literature Since 2005
The surgical procedures used included total and partial laryngectomy (7 cases were treated using CO2 lasers; the others underwent robotic partial laryngectomy48). As concluded by Woodruff and Senie (1991)44 and Ferlito et al (2005, 2006),42,45 surgery remains the mainstream treatment for atypical laryngeal carcinoid today. Recently, transoral robotic partial laryngectomy has become possible.48 The principal advantages of robotic partial laryngectomy (compared to the traditional open approaches) are preservation of laryngeal function and elimination of the need for permanent tracheostomy.48 However, such surgery is expensive and few hospitals have the sophisticated equipment required. Thus, CO2 laser treatment delivered under laryngomicroscopic guidance remains the principal surgical modality for the treatment of early-stage atypical laryngeal carcinoid patients.9,32,41,49,55
Follow-up information was available for 39 (92.9%) of the 42 patients. Nineteen (48.7%) developed regional recurrences, 12 (30.8%) distant metastases, and 6 (15.4%) both cutaneous and subcutaneous metastases. Overall, 11 patients (28.2%) remained alive and free of disease; 16 41.0%) died from the disease, 2 (5.1%) died from other diseases, 8 (20.5%) were alive with disease, and 1 (2.6%) was lost to follow-up. The 5- and 10-year survival rates were 57.0% and 15.0%, respectively (Figure 8). The 5-year rate was similar to that reported by Woodruff and Senie (1991).44 However, the 10-year rate was lower than that reported by the cited authors.44 We found that neither the treatment modality used nor the development of local recurrence affected outcomes (both P values >0.05). The development of distant metastasis significantly (negatively) affected survival. The estimated mean survival time of patients with distant metastases was poorer than that of others (102.0 vs 41.3 months, P < 0.01).
FIGURE 8.
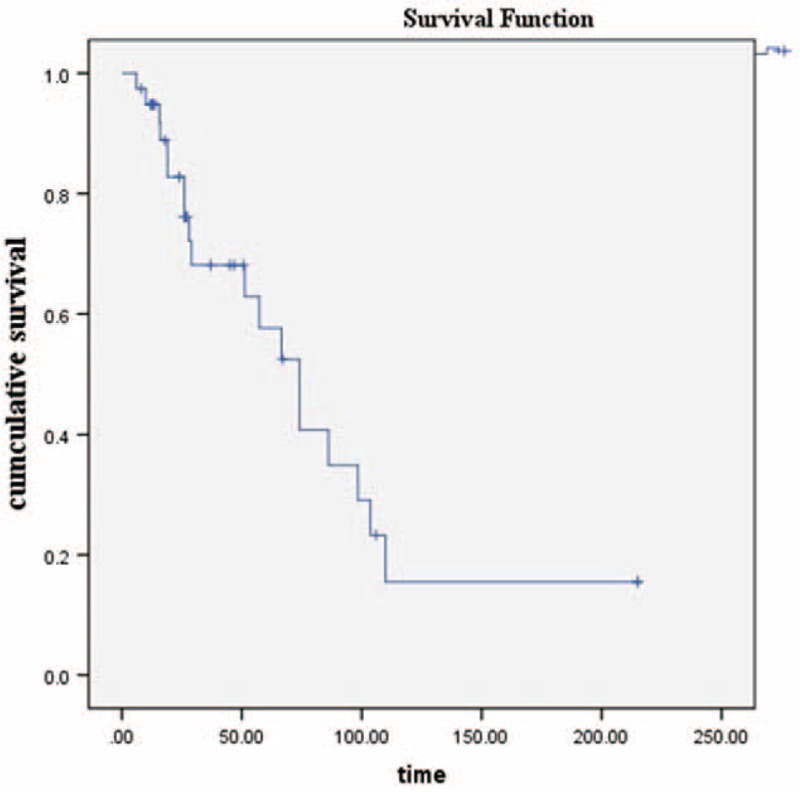
The 5- and 10-year survival rates of 39 patients diagnosed since our last review in 2005 were 57.0% and 15.0%, respectively.
We found that the rates of cutaneous and subcutaneous metastasis in these patients were lower (22%) than those reported by Woodruff and Senie (1991).44 We searched the English language literature for information on cutaneous and subcutaneous metastases in such patients. We found data on 48 such patients (including our present case) (Table 3 ).6,8,32,34–36,40,44,54,56,57 To the best of our knowledge, approximately 392 cases of atypical laryngeal carcinoids have been reported in the English language literature, combining the data of Ferlito et al42,45 with those of the present review. The frequency of metastasis in such patients is approximately 12.2%; this incidence may fall as more data accumulate. Thirty patients (62.5%) with such metastases exhibited contemporaneous lymph node invasion. Cutaneous and subcutaneous metastases were always accompanied by distant metastases to other sites, including bone, lung, heart, liver, gallbladder, pancreas, bowel, kidney, ureter, testis, thyroid, diaphragm, and rectus muscle. The principal symptom was pain. Of the 42 patients (87.5%) for whom follow-up data were available, the 3-, 5-, and 10-year survival rates were 44.0%, 22.0%, and 13.0%, respectively (Figure 9).
TABLE 2 (Continued).
Data on 42 New Cases Described in the English Language Literature Since 2005
FIGURE 9.
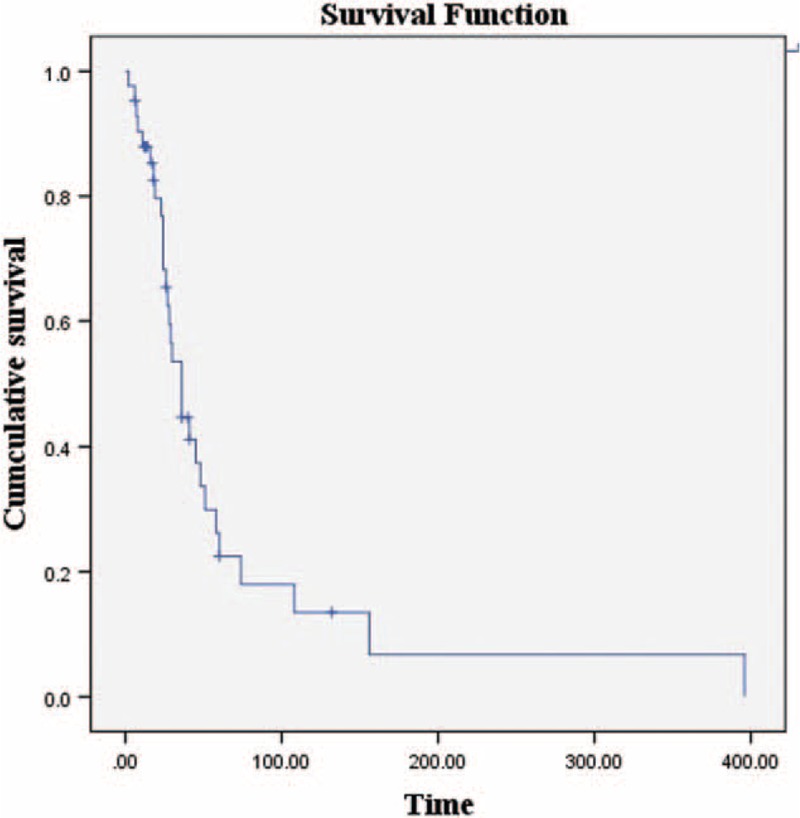
The 3-, 5-, and 10-year survival rates of patients described in the English language literature with cutaneous and subcutaneous metastases from atypical laryngeal carcinoids were 44.0%, 22.0%, and 13.0%, respectively.
TABLE 3.
Data on 48 Patients Described in the English Language Literature With Cutaneous and Subcutaneous Metastases From Atypical Laryngeal Carcinoids
TABLE 3 (Continued).
Data on 48 Patients Described in the English Language Literature With Cutaneous and Subcutaneous Metastases From Atypical Laryngeal Carcinoids
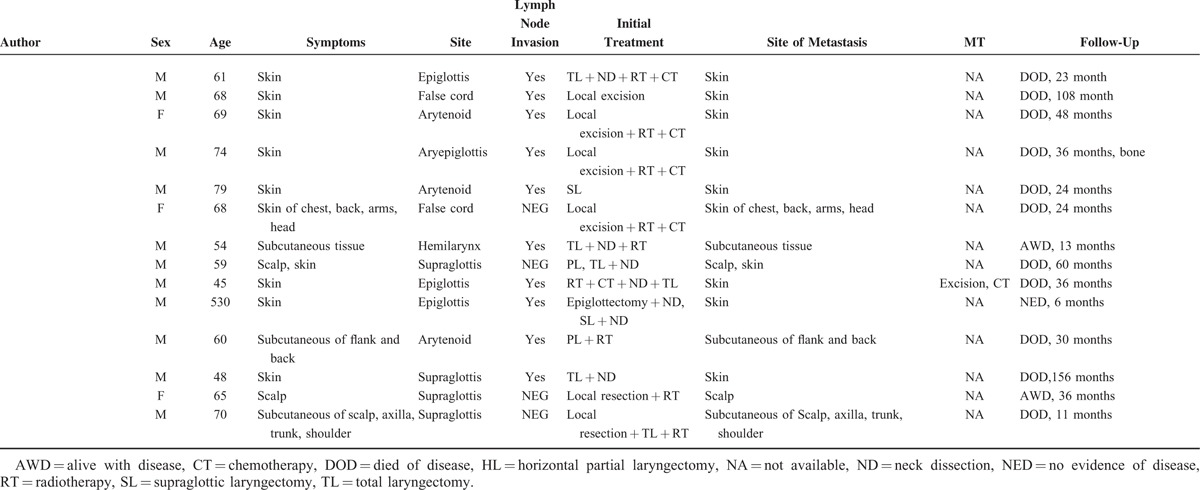
No satisfactory treatment for cutaneous and subcutaneous metastases of atypical laryngeal carcinoids has yet been described. Descriptions of treatments are rare.6,36,40,4,56,58,59 In 1989, Ferlito and Friedmann proposed that painful skin/subcutaneous metastases should be treated surgically.44 van der Laan et al (2012) suggested that salvage surgery, including palliative metastasectomy, had an important role in the treatment of laryngeal NEC. In addition, the overall survival level was reasonable. The cited authors described 3 patients with atypical laryngeal carcinoids who developed cutaneous and subcutaneous metastases; 1 died of disease 74 months after primary treatment (total laryngectomy); 1 received miltefosine after metastasis was diagnosed and died of disease 28 months after primary treatment (horizontal laryngectomy); and 1 underwent total laryngectomy after metastasis was diagnosed and was alive with disease 26 months after primary treatment (supraglottic laryngectomy).41 Simpson et al (2009)7 reported on an 82-year-old patient who presented with multiple exquisitely tender skin lesions. The patient had undergone resection of an atypical laryngeal carcinoid 33 years prior, and 2 further endoscopic laser resections (with adjuvant radiotherapy) due to local recurrence 2 years prior. The multiple skin metastases were treated using a CO2 laser; palliation was effective. However, the patient died from unrelated causes 8 months after the final presentation.6 Overholt et al (1995)36 described a patient with skin metastases who underwent chemotherapy. The patient died of disease 26 months after primary treatment. It was thus suggested that chemotherapy was ineffective. Gripp et al (1995)58 used somatostatin to treat skin metastases from atypical laryngeal carcinoids. However, the drug was no better than other chemotherapies.58 Electrochemotherapy is useful to palliate cutaneous and subcutaneous metastases arising from some other malignant tumors.60 Such management included intravenous bleomycin (15,000 IU/m2); the electrodes were placed under general anesthesia. The overall response rate was 66.6%. Patients with less than 10 nodules and masses smaller than 2 cm in diameter benefited the most.60 In our present case, multiple cutaneous and subcutaneous metastases developed 6 months after supraglottic laryngectomy. The tested treatments were unsatisfactory. Pain was relieved only via intrathecal drug delivery.
In conclusion, atypical laryngeal carcinoids are rare, and the prognoses are poor. Prognostic factors include the level of p53, HPV status, certain hypoxic markers, and distant metastasis. The rate of development of cutaneous and subcutaneous metastases was 12.2%. No optimal treatment for such metastases has yet been defined.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (grant nos. 81172562 and 81372903).
Footnotes
Abbreviations: CMV = cytomegalovirus, EBV = Epstein–Barr virus, FDG = [18F]-fluoro-2-deoxy-d-glucose, GLUT-1 = glucose transporter-1, HIF-1α = hypoxia-inducible factor-1α, HPV = human papilloma virus, NEC = neuroendocrine carcinomas, PET/CT = positron emission tomography/computed tomography, PI3K/Akt = phosphatidylinositol 3-kinase/protein kinase B.
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/3ocKWB.
This research was supported by the National Natural Science Foundation of China (grant nos. 81172562 and 81372903).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Barnes L. Barnes L, Eveson JW, Reichart P, Sidransky D. Neuroendocrine tumours. Pathology and Genetics. Head and Neck Tumours. World Health Organization Classification of Tumours. Lyon: IARC Press; 2005. 135–139. [Google Scholar]
- 2.van der Laan TP, Plaat BE, van der Laan BF, et al. Clinical recommendations on the treatment of neuroendocrine carcinoma of the larynx: a meta-analysis of 436 reported cases. Head Neck 2015; 37:707–715. [DOI] [PubMed] [Google Scholar]
- 3.Ferlito A, Lewis JS, Jr, Rinaldo A. The evolving management of laryngeal neuroendocrine carcinomas. Eur Arch Otorhinolaryngol 2011; 268:1247–1248. [DOI] [PubMed] [Google Scholar]
- 4.Ferlito A, Rinaldo A. The spectrum of endocrinocarcinomas of the larynx. Oral Oncol 2005; 41:878–883. [DOI] [PubMed] [Google Scholar]
- 5.Ferlito A, Barnes L, Rinaldo A, et al. A review of neuroendocrine neoplasms of the larynx: update on diagnosis and treatment. J Laryngol Otol 1998; 112:827–834. [DOI] [PubMed] [Google Scholar]
- 6.Simpson LK, Ostlere LS, Harland C, et al. Treatment with carbon dioxide laser of painful skin metastases from a laryngeal neuroendocrine carcinoma. Clin Exp Dermatol 2009; 34:e873–875. [DOI] [PubMed] [Google Scholar]
- 7.Rosai J. Respiratory tract. Rosai and Ackerman's Surgical Pathology. 9th Ed. Elsevier, 2005; 1:411–12. [Google Scholar]
- 8.AL H, K A, K A, et al. Primary laryngeal neuroendocrine carcinoma - a rare entity with deviant clinical presentation. J Clin Diagn Res 2014; 8:FD07–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanazawa T, Nokubi M, Takeoda K, et al. Atypical carcinoid of the larynx and expressions of proteins associated with molecular targeted therapy. Auris Nasus Larynx 2011; 38:123–126. [DOI] [PubMed] [Google Scholar]
- 10.Liu JC, Ridge JA, Brizel DM, et al. Current status of clinical trials in head and neck cancer 2014. Otolaryngol Head Neck Surg 2015; 152:410–417. [DOI] [PubMed] [Google Scholar]
- 11.Chen WH, Lecaros RL, Tseng YC, et al. Nanoparticle delivery of HIF1 (siRNA combined with photodynamic therapy as a potential treatment strategy for head-and-neck cancer. Cancer Lett 2015; 359:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shida T, Kishimoto T, Furuya M, et al. Expression of an activated mammalian target of rapamycin (mTOR) in gastroenteropancreatic neuroendocrine tumors. Cancer Chemother Pharmacol 2010; 65:889–893. [DOI] [PubMed] [Google Scholar]
- 13.Volante M, Birocco N, Gatti G, et al. Extrapulmonary neuroendocrine small and large cell carcinomas: a review of controversial diagnostic and therapeutic issues. Hum Pathol 2014; 45:665–673. [DOI] [PubMed] [Google Scholar]
- 14.Vlachostergios PJ, Papandreou CN. Targeting neuroendocrine prostate cancer: molecular and clinical perspectives. Front Oncol 2015; 5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ying HF, Bao YY, Zhou SH, et al. Submucosal small-cell neuroendocrine carcinoma of the larynx detected using (18)F-fluorodeoxyglucose positron emission tomography/computed tomography: a case report and review of the literature. Oncol Lett 2014; 8:1065–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miki K, Orita Y, Nose S, et al. Neuroendocrine carcinoma of the larynx presenting as a primary unknown carcinoma. Auris Nasus Larynx 2012; 39:98–102. [DOI] [PubMed] [Google Scholar]
- 17.Chun BJ, Yoo IR, Joo YH, et al. The efficacy of 18F-FDG PET/CT imaging for extracapsular spread of the laryngeal squamous cell carcinoma. Head Neck 2016; 38:290–293. [DOI] [PubMed] [Google Scholar]
- 18.Roh JL, Park JP, Kim JS, et al. 18Ffluorodeoxyglucose PET/CT in head and neck squamous cell carcinoma with negative neck palpation findings: a prospective study. Radiology 2014; 271:153–161. [DOI] [PubMed] [Google Scholar]
- 19.Zhao K, Luo XM, Zhou SH, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography as an effective diagnostic workup in cervical metastasis of carcinoma from an unknown primary tumor. Cancer Biother Radiopharm 2012; 27:685–693. [DOI] [PubMed] [Google Scholar]
- 20.Zhao K, Yang SY, Zhou SH, et al. Fluorodeoxyglucose uptake in laryngeal carcinoma is associated with the expression of glucose transporter-1 and hypoxia-inducible-factor-1α and the phosphoinositide 3-kinase/protein kinase B pathway. Oncol Lett 2014; 7:984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Berkel A, Rao JU, Kusters B, et al. Correlation between in vivo 18F-FDG PET and immunohistochemical markers of glucose uptake and metabolism in pheochromocytoma and paraganglioma. J Nucl Med 2014; 55:1253–1259. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi M, Kaida H, Kawahara A, et al. The relationship between GLUT-1 and vascular endothelial growth factor expression and 18F-FDG uptake in esophageal squamous cell cancer patients. Clin Nucl Med 2012; 37:447–452. [DOI] [PubMed] [Google Scholar]
- 23.Li SJ, Guo W, Ren GX, et al. Expression of Glut-1 in primary and recurrent head and neck squamous cell carcinomas, and compared with 2-[18F]fluoro-2-deoxy-D-glucose accumulation in positron emission tomography. Br J Oral Maxillofac Surg 2008; 46:180–186. [DOI] [PubMed] [Google Scholar]
- 24.Huang XQ, Chen X, Xie XX, et al. Co-expression of CD147 and GLUT-1 indicates radiation resistance and poor prognosis in cervical squamous cell carcinoma. Int J Clin Exp Pathol 2014; 7:1651–1666. [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada T, Uchida M, Kwang-Lee K, et al. Correlation of metabolism/hypoxia markers and fluorodeoxyglucose uptake in oral squamous cell carcinomas. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113:464–471. [DOI] [PubMed] [Google Scholar]
- 26.Ozbudak IH, Shilo K, Tavora F, et al. Glucose transporter-1 in pulmonary neuroendocrine carcinomas:expression and survival analysis. Mod Pathol 2009; 22:633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chai L, Ying HF, Wu TT, et al. Clinical features and hypoxic marker expression of primary sinonasal and laryngeal small-cell neuroendocrine carcinoma: a small case series. World J Surg Oncol 2014; 12:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song YS, Lee WW, Chung JH, et al. Correlation between FDG uptake and glucose transporter type 1 expression in neuroendocrine tumors of the lung. Lung Cancer 2008; 61:54–60. [DOI] [PubMed] [Google Scholar]
- 29.Zhou SH, Fan J, Chen XM, et al. Inhibition of cell proliferation and glucose uptake in human laryngeal carcinoma cells by antisense oligonucleotides against glucose transporter-1. Head Neck 2009; 31:1624–1633. [DOI] [PubMed] [Google Scholar]
- 30.Yan SX, Luo XM, Zhou SH, et al. Effect of antisense oligodeoxynucleotides glucose transporter-1 on enhancement of radiosensitivity of laryngeal carcinoma. Int J Med Sci 2013; 10:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giordano G, Corcione L, Giordano D, et al. Primary moderately differentiated neuroendocrine carcinoma (atypical carcinoid) of the larynx: a case report with immunohistochemical and molecular study. Auris Nasus Larynx 2009; 36:228–231. [DOI] [PubMed] [Google Scholar]
- 32.Halmos GB, van der Laan TP, van Hemel BM, et al. Is human papillomavirus involved in laryngeal neuroendocrine carcinoma? Eur Arch Otorhinolaryngol 2013; 270:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erler BS, Presby MM, Finch M, et al. CD117, Ki-67, and p53 predict survival in neuroendocrine carcinomas, but not within the subgroup of small cell lung carcinoma. Tumour Biol 2011; 32:107–111. [DOI] [PubMed] [Google Scholar]
- 34.Chung JH, Lee SS, Shim YS, et al. A study of moderately differentiated neuroendocrine carcinomas of the larynx and an examination of non-neoplastic larynx tissue for neuroendocrine cells. Laryngoscope 2004; 114:1264–1270. [DOI] [PubMed] [Google Scholar]
- 35.McCluggage WG, Cameron CH, Arthur K, et al. Atypical carcinoid tumor of the larynx: an immunohistochemical, ultrastructural, and flow cytometric analysis. Ultrastruct Pathol 1997; 21:431–438. [DOI] [PubMed] [Google Scholar]
- 36.Overholt SM, Donovan DT, Schwartz MR, et al. Neuroendocrine neoplasms of the larynx. Laryngoscope 1995; 105:789–794. [DOI] [PubMed] [Google Scholar]
- 37.Hafner C, Houben R, Baeurle A, et al. Activation of the PI3K/AKT pathway in Merkel cell carcinoma. PLoS One 2012; 7:e31255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leung R, Lang B, Wong H, et al. Advances in the systemic treatment of neuroendocrine tumors in the era of molecular therapy. Anticancer Agents Med Chem 2013; 13:382–388. [PubMed] [Google Scholar]
- 39.Kaira K, Murakami H, Endo M, et al. Biological correlation of 18F-FDG uptake on PET in pulmonary neuroendocrine tumors. Anticancer Res 2013; 33:4219–4228. [PubMed] [Google Scholar]
- 40.van der Laan TP, van der Laan BF, Plaat BE, et al. Neuroendocrine carcinoma of the larynx – an extraordinary malignancy with high recurrence rates and long survival: our experience in 11 patients. Clin Otolaryngol 2012; 37:63–66. [DOI] [PubMed] [Google Scholar]
- 41.Carić T, Bilić M, Bilić LK, et al. Neuroendocrine tumors of larynx – two case reports and literature review. Coll Antropol 2012; 36:173–178. [PubMed] [Google Scholar]
- 42.Ferlito A, Devaney KO, Rinaldo A. Neuroendocrine neoplasms of the larynx: advances in identification, understanding, and management. Oral Oncol 2006; 42:770–788. [DOI] [PubMed] [Google Scholar]
- 43.Soga J. Carcinoids and their variant endocrinomas. An analysis of 11842 reported cases. J Exp Clin Cancer Res 2003; 22:517–530. [PubMed] [Google Scholar]
- 44.Woodruff JM, Senie RT. Atypical carcinoid tumor of the larynx. A critical review of the literature. ORL J Otorhinolaryngol Relat Spec 1991; 53:194–209. [DOI] [PubMed] [Google Scholar]
- 45.Ferlito A, Friedmann A. Review of neuroendocrine carcinomas of the larynx. AnnOtol Rhinol Laryngol 1989; 98:780–790. [DOI] [PubMed] [Google Scholar]
- 46.Ferlito A, Silver CE, Bradford CR, et al. Neuroendocrine neoplasms of the larynx: an overview. Head Neck 2009; 31:1634–1646. [DOI] [PubMed] [Google Scholar]
- 47.Kumar PD, Simha NV, Sreenivas N. Atypical carcinoid of larynx: a case study and a brief review. J Clin Diagn Res 2014; 8:KD03–KD04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muderris T, Bercin S, Sevil E, et al. Transoral robotic surgery for atypical carcinoid tumor of the larynx. J Craniofac Surg 2013; 24:1996–1999. [DOI] [PubMed] [Google Scholar]
- 49.Davies-Husband CR, Montgomery P, Premachandra D, et al. Primary, combined, atypical carcinoid and squamous cell carcinoma of the larynx: a new variety of composite tumour. J Laryngol Otol 2010; 124:226–229. [DOI] [PubMed] [Google Scholar]
- 50.Zhang M, Zhou L, Li C, et al. Moderately differentiated neuroendocrine carcinoma of the larynx. Acta Otolaryngol 2010; 130:498–502. [DOI] [PubMed] [Google Scholar]
- 51.Seshamani M, Einhorn E, Mirza N. Atypical carcinoid of the larynx and potential complications of the carcinoid syndrome: a case report. Ear Nose Throat J 2009; 88:E1. [PubMed] [Google Scholar]
- 52.Chung EJ, Baek SK, Kwon SY, et al. Moderately differentiated euroendocrine carcinoma of the larynx. Clin Exp Otorhinolaryngol 2008; 1:217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Capelli M, Bertino G, Morbini P, et al. Neuroendocrine carcinomas of the upper airways: a small case series with histopathological considerations. Tumori 2007; 93:499–503. [DOI] [PubMed] [Google Scholar]
- 54.Gillenwater A, Lewin J, Roberts D, et al. Moderately differentiated neuroendocrine carcinoma (atypical carcinoid) of the larynx: a clinically aggressive tumor. Laryngoscope 2005; 115:1191–1195. [DOI] [PubMed] [Google Scholar]
- 55.Chang KP, Lee LY, Yeh AR, et al. Endoscopic CO2 laser surgery for an atypical carcinoid tumor of the epiglottis masquerading as a supraglottic cyst. Head Neck 2005; 27:1004–1007. [DOI] [PubMed] [Google Scholar]
- 56.Machens A, Holzhausen HJ, Dralle H. Minimally invasive surgery for recurrent neuroendocrine carcinoma of the supraglottic larynx. Eur Arch Otorhinolaryngol 1999; 256:242–246. [DOI] [PubMed] [Google Scholar]
- 57.Ereño C, Lopez JI, Sanchez JM. Atypical carcinoid of larynx: presentation with scalp metastases. J Laryngol Otol 1997; 111:89–91. [DOI] [PubMed] [Google Scholar]
- 58.Gripp FM, Risse EK, Leverstein H, et al. Neuroendocrine neoplasms of the larynx. Importance of the correct diagnosis and differences between atypical carcinoid tumors and small-cell neuroendocrine carcinoma. Eur Arch Otorhinolaryngol 1995; 252:280–286. [DOI] [PubMed] [Google Scholar]
- 59.Watters GW, Molyneux AJ. Atypical carcinoid tumour of the larynx. J Laryngol Otol 1995; 109:455–458. [DOI] [PubMed] [Google Scholar]
- 60.Solari N, Spagnolo F, Ponte E, et al. Electrochemotherapy for the management of cutaneous and subcutaneous metastasis: a series of 39 patients treated with palliative intent. J Surg Oncol 2014; 109:270–274. [DOI] [PubMed] [Google Scholar]


