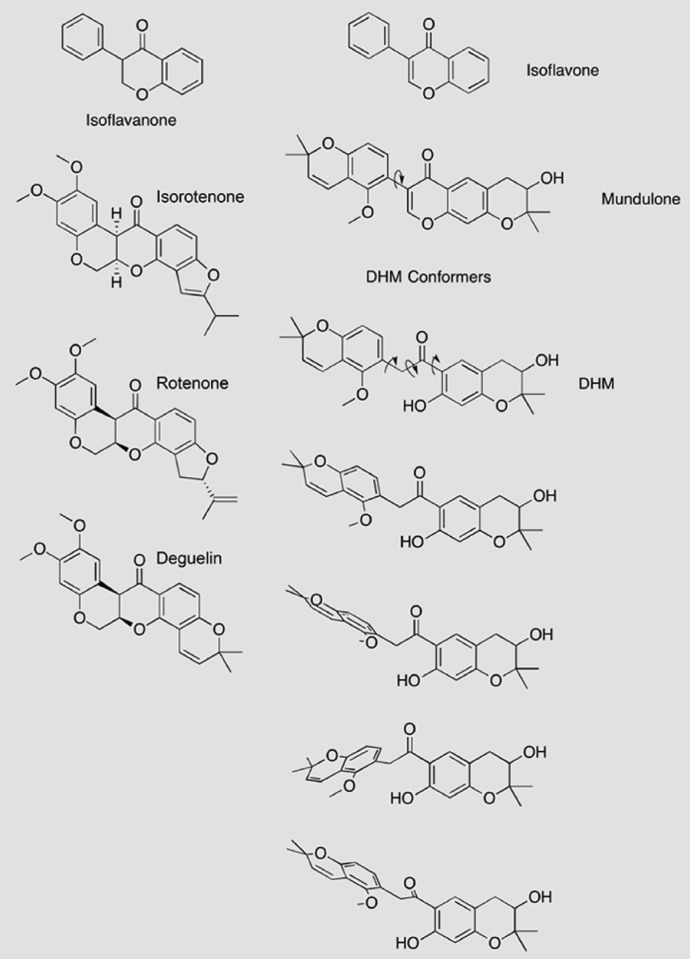
Fig. 6.
Structural features of DHM and related compounds. The rotenoids (isorotenone, rotenone, and deguelin) are relatively rigid, planar compounds with isoflavanone-like cores. In contrast, mundulone, a true isoflavone, and DHM both lack the isoflavanone core, which allows for increased flexibility and the predicted ability to adopt multiple conformations. Mundulone may rotate around one central carbon-carbon bound, indicated by the arrow. The core of DHM is a 2-phenylacetophenone that would permit rotation about the three indicated carbon-carbon bonds, strongly suggesting that DHM is the most flexible compound of the five compounds studied. Representative conformers of DHM are shown to illustrate the predicted flexibility of the compound [conformational analysis was done with the Merck Molecular Force Field (MMFF) force field, iSpartan (www.wavefun.com); structures were drawn with ChemDraw Pro (Perkin Elmer, Waltham, MA)].