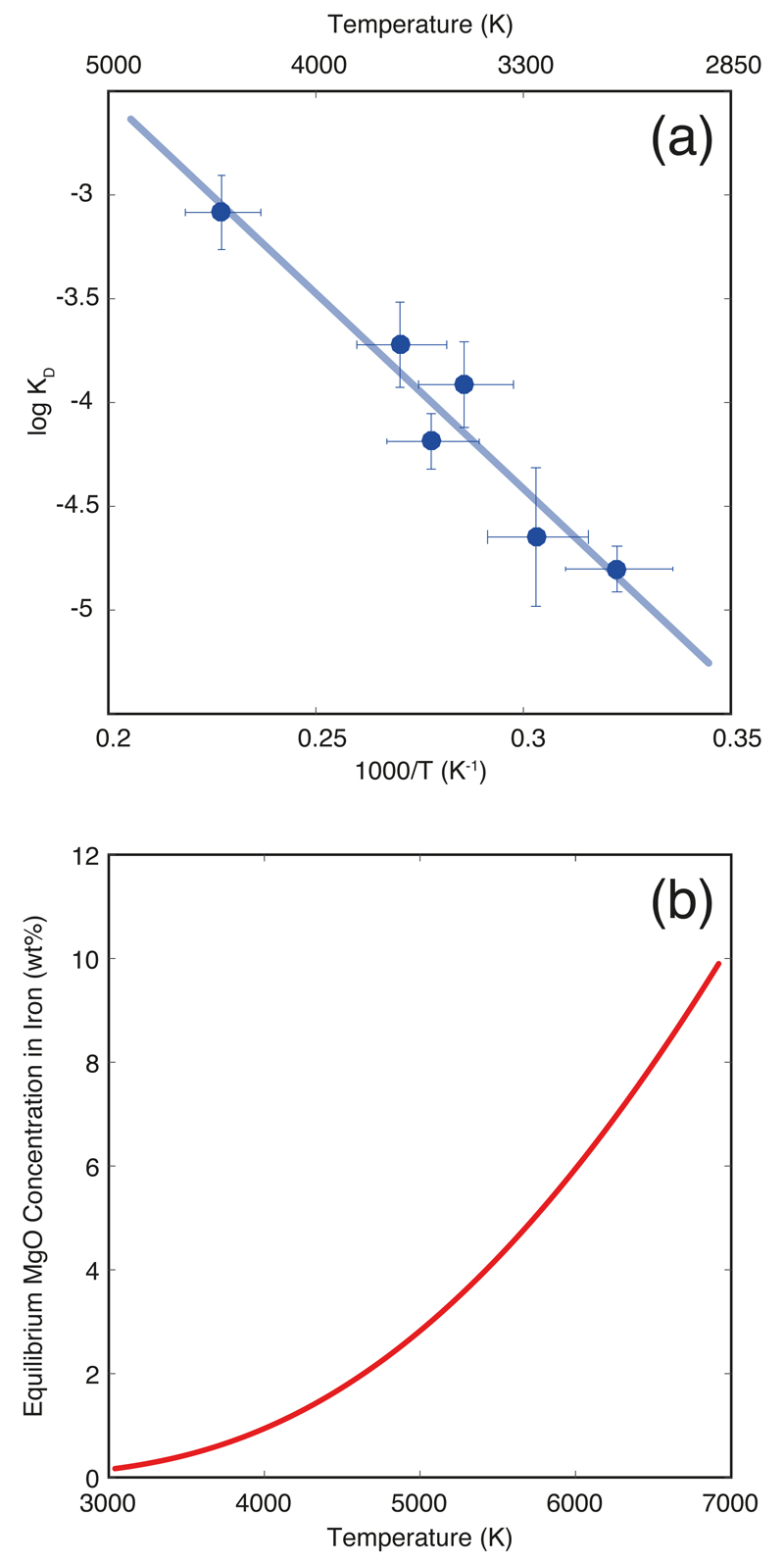
Figure 1. Magnesium solubility in metallic iron melt at high pressure and temperature.
(a) Equilibrium constant for MgO dissolution in iron as a function of reciprocal temperature (Eq. 2). The experimental data is from Extended Data Table 1. The line corresponds to the least-squares linear fit to the data. A comparison with extrapolation from DFT calculations4 is shown in Extended Data Fig. 2. (b) The resulting MgO concentration in iron in equilibrium with pyrolite as a function of temperature. This is obtained by rewriting (Eq. 2) to obtain , where T is temperature in K, and then converting Mg molar fractions to MgO weight fractions. This is the saturation MgO concentration in the core at a given temperature, and shows that for a present-day CMB temperature of 4100 K, the core cannot contain more than 1.1 wt.% MgO: any MgO dissolved in the core (during core formation) in excess of that value must have exsolved. For an extended version of this graph, see Extended Data Fig. 6.