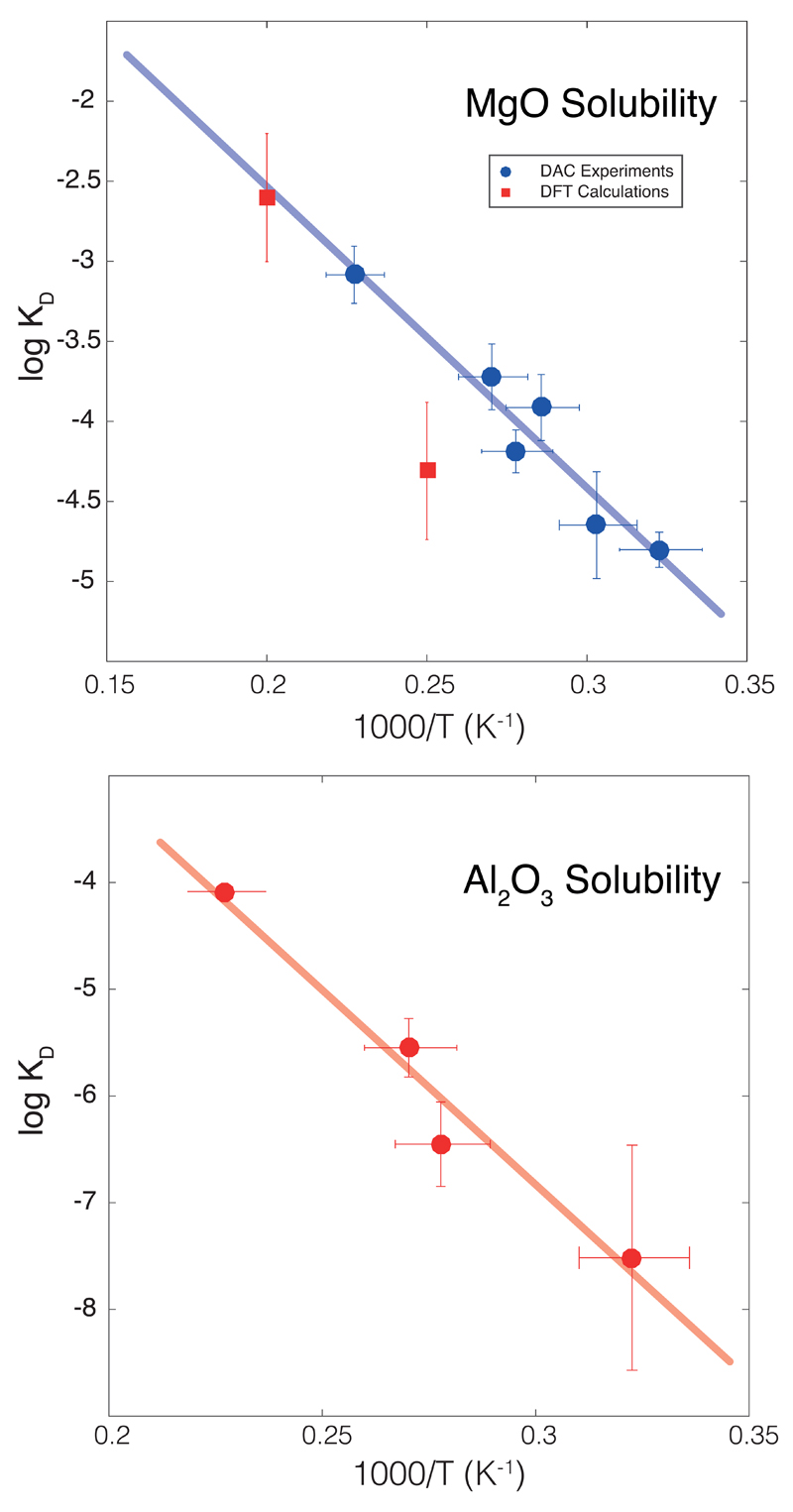
Extended Data Figure 2. Magnesium and aluminum solubility in metallic iron melt at high pressure and temperature.
(top) Equilibrium constant for MgO dissolution in molten iron as a function of reciprocal temperature. The circles correspond to the experimental data (Extended Data Table 1) and error bars to standard error, while the squares correspond to the low-temperature extrapolation of DFT calculations4. The thick line corresponds to the least-squares linear fit to the experimental data alone (Fig. 1); it shows the remarkable agreement between the theoretical and experimental datasets, especially at high temperature where the theoretical dataset (which is extrapolated from higher temperatures) is the least influenced by extrapolation. (bottom) Equilibrium constant for Al2O3 dissolution (see Methods) in molten iron as a function of reciprocal temperature. The circles correspond to the experimental data (Extended Data Table 1) and error bars to standard error. The thick line corresponds to the least-squares linear fit to the data (R2=0.92), and we find .