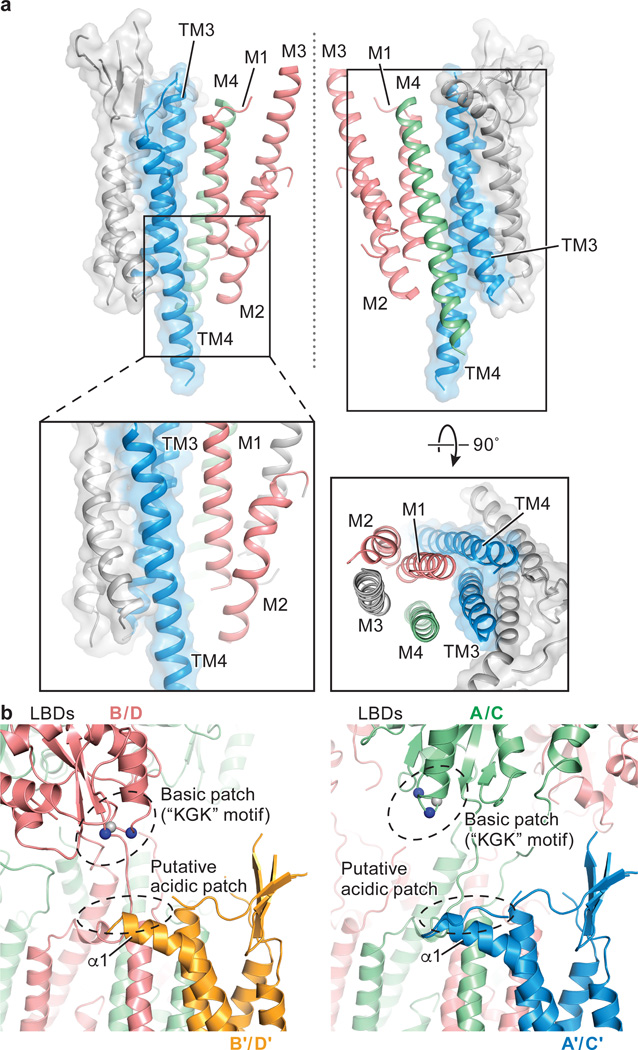
Figure 3. Interactions between GluA2 receptor and TARP γ2.
a, TM-TM interactions between TARP γ2 and receptor at A/C positions. TARP γ2 is shown in ribbon diagram in transparent surface representation, while helices participating in interactions with receptor are highlighted in color. The central axis of the ion channel pore is indicated by a dashed line. A close-up view emphasizes likely hydrophobic interactions between TARP and receptor, whereas a top-down view illustrates that all TM helices but M3 of the receptor interact with TARP. TM helices from selected receptor subunits were omitted for clarity. b, Interactions between TARP γ2 ECD and receptor LBD at the A/C positions differ from interactions at the B/D positions. TARP γ2 ECD and receptor LBD are in closer proximity in the B/D positions (right) than in the A/C positions (left). The Cα atoms of the “KGK” motif (697-699) are shown as spheres. Lysine and glycine Cαs are colored in blue and grey, respectively.