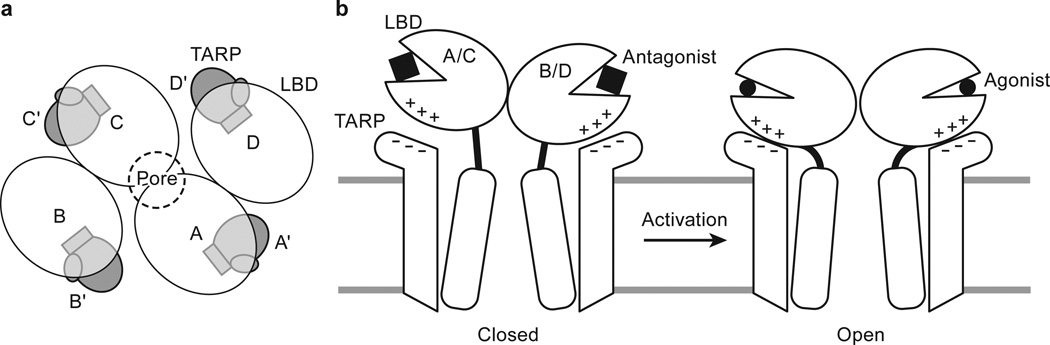
Figure 4. Mechanism for TARP γ2 modulation of receptor gating.
a, TARP γ2 subunits resemble partially opened palms and are positioned “underneath” receptor LBDs in the antagonist-bound state. b, During receptor activation, TARP “palms” engage with receptor LBD to stabilize intra-dimer and inter-dimer interfaces, modulating receptor activation, deactivation and desensitization. An extracellular loop of TARP γ2 rich in negatively charged residues facilitates the motion of receptor LBD lower lobe rich in positively charged residues upon receptor activation, whereas TARP γ2 TMD directly interacts with receptor TMD including the pore-lining M2 helices, leading to modulation of receptor pore properties.