Abstract
Minimally invasive surgery has been cautiously introduced in surgical oncology over the last two decades due to a concern of compromised oncological outcomes. Recently, it has been adopted in liver surgery for colorectal metastases. Colorectal cancer is a major cause of cancer-related death in the USA. In addition, liver metastasis is the most common site of distant disease and its resection improves survival. While open resection was the standard of care, laparoscopic liver surgery has become the standard of care for minor liver resections. Laparoscopic liver surgery provides equivalent oncological outcomes with better perioperative results compared to open liver surgery. Robotic liver surgery has been introduced as it is believed to overcome some of the limitations of laparoscopy. Finally, laparoscopic radio-frequency ablation and microwave coagulation can be used as adjuncts in minimally invasive surgery to complement or replace surgical resection when not possible.
Keywords: Minimally invasive liver surgery, Laparoscopic liver surgery, Robotic liver surgery, Laparoscopic radio-frequency ablation, Laparoscopic microwave ablation, Colorectal cancer, Colorectal cancer liver metastasis
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer-related death in the USA with an estimated incidence of 132, 700 new cases and 49,700 deaths in 2015 [1]. Twenty to twenty-five percent of patients with CRC present with synchronous metastasis, and approximately 50–60 % will develop metastasis during the evolution of the disease. The liver is the most common site accounting for 80 % of stage IV patients and 40 % as the only site of distant disease [2–4]. Although the use of multidrug chemotherapy has improved tumor response, the median survival for patients with unresectable disease is poor and the 5-year survival is null [5–10]. Resection, when feasible, is the gold standard of care as it confers a higher chance of cure and, thus, better long-term survival [11]. Resection of colorectal cancer liver metastasis (CRCLM) can improve 5-year survival to 34–60 % [12–15, 16••, 17]. In addition to expanding the resectability criteria for CRCLM, there is an evolution in the techniques adopted [18, 19]. While open surgical resection was the mainstay of treatment, minimally invasive surgery has been slowly adopted in liver surgery over the last two decades. Laparoscopic liver resection (LLR) has been increasingly performed and accepted as a safe and feasible procedure and considered a standard of care for minor liver resections. Short- and long-term oncological outcomes seem to be similar to open liver resection (OLR) in CRCLM, which legitimized the use of this approach more often nowadays. In addition, robotic surgery has been introduced in this field but is still considered a new technique and is under investigation. Robotic surgery overcomes some of the limitations of laparoscopy as it allows three-dimensional visualization, eliminates surgeon tremors, and allows better articulation. In this review, we will discuss the role of laparoscopy and robotic surgery in the management of CRCLM as well as adjunct treatments such as laparoscopic radio-frequency ablation (RFA) and microwave coagulation (MWC).
Laparoscopic Liver Resection
Background
Gagner et al. performed the first laparoscopic liver resection for benign disease in 1992 [20]. In 1993, Azagra et al. and Talamini reported the first anatomical liver resection, a left lateral segmentectomy for symptomatic hepatic adenoma [21, 22] while Wayand and Woisetschläger performed the first laparoscopic liver resection for colorectal metastasis [23]. Since then, laparoscopy has been used more often for the resection of both benign and malignant diseases of the liver. Two international consensus conferences were held to discuss the role of LLR: one in Louisville, USA, in 2008, and the other in Morioka, Japan, in 2014. This second international consensus conference concluded that minor LLRs became the standard of care while major LLRs were considered an innovative technique still under investigation [24, 25]. Minor LLR involves the resection of two or fewer Couinaud segments excluding the posterior-superior segments that pose a higher surgical challenge [24, 26].
While the adoption of laparoscopy has been fast for surgical procedures like cholecystectomy, hernia repair, adrenalectomy, bariatric surgery, and splenectomy, it has been slowly introduced in the surgical oncology field. This stems from the concern of inadequate margins or lymph node sampling, tumor seeding, missing small metastases, and poor pathological and oncological outcomes. In addition to the complexity of laparoscopic liver surgery, concerns of air embolism have added an additional hurdle to adopting minimally invasive liver surgery. It was not until 2000 when Cherqui et al. [27] published a feasibility study of 30 patients undergoing LLR for both benign and malignant diseases of the liver (both primary and metastatic) that surgeons started accepting this technique as an alternative to open surgery. Since then, an increasing number of centers started to perform minor and major LLRs. Nearly 10,000 LLRs have been reported in the literature, showing the widely acceptance of this technique and its safety [28].
Technique
Laparoscopic minor liver resection is considered the standard of care and is being adopted by an increasing number of surgical centers. The ideal indications for LLR are in patients with single and peripheral lesions that measure less than 5 cm. Major LLR should be performed by a highly experienced surgeon in the field of minimally invasive surgery. A LLR technique varies across surgeons’ preferences. Patients are usually placed in a supine reverse Trendelenburg position. For minor liver resections, we prefer the placement of two 12-mm trocars (for camera and energy/stapler device) and two 5-mm trocars. Trocar placement varies according to the location of the segment or section to be resected. Placement of an umbilical tape around the hepatic pedicle for a potential Pringle maneuver should be considered if parenchymal bleeding is anticipated. Intraoperative ultrasound should be routinely used to define tumor location and vascular anatomy before hepatic resection. Hepatic transection is usually achieved with a combination of energy devices. The authors’ preferences are monopolar cautery, laparoscopic ultrasonic scalpel (HARMONIC ACE®+ Shears, Ethicon Endo-Surgery, Cincinnati, OH), and the Aquamantys Endo Dissecting Bipolar Sealer 8.7 (Medtronic, Minneapolis, MN) for hemostasis of the transected margin. If small vascular or biliary pedicles are identified, these can be clipped and transected. For larger pedicles, laparoscopic linear vascular stapler devices should be used. Revision of the transected margin for hemostasis and prevention of biliary leak or biloma formation is essential. The authors do not routinely place drains unless there is a concern for biliary leaks or bleeding. The specimen is extracted inside an endoscopic bag enlarging one of the 12-mm incisions as needed.
It is relevant to highlight the importance of intraoperative ultrasound (IOUS) in the detection of colorectal cancer liver metastases in patients undergoing liver surgery. It has been reported that IOUS can detect additional liver lesions and change surgical management in 2–18 % of cases [29–31]. Even in the current era of high-quality, modern cross-sectional imaging (CT, MRI, PET-CT), van Vledder et al. showed that IOUS detected additional liver metastases in 10 % of patients in a series of 213 patients undergoing liver surgery for CRCLM [30]. Furthermore, we consider IOUS as a fundamental tool to identify and map out not only liver lesions but also vascular and biliary duct anatomy, which is crucial when performing minimally invasive surgery. Laparoscopic liver ultrasonography has some technical limitations when compared to open surgery and requires a learning curve. Adequate liver mobilization to facilitate tissue apposition of the laparoscopic probe in the liver surface is sometimes necessary. Laparoscopic IOUS probes are linear and require 10- or 12-mm trocars to be introduced. The movement of the linear laparoscopic IOUS probe is limited when compared to open surgery, and the insertion of the laparoscopic probe through different trocar sites is sometimes needed. The authors also recommend the use of four-way laparoscopic probes that allow to reach more posterior and uneven liver surfaces. Despite these limitations, Viganò et al. in a recent series of 65 patients demonstrated that laparoscopic liver ultrasound was able to detect 18.5 % more lesions than previously found in preoperative imaging and it is comparable to the open technique [32].
Outcomes
There are no available randomized controlled trials (RCTs) to compare safety and short- and long-term clinical and oncological outcomes between LLR and OLR for CRCLM. Two RCTs are being performed to compare LLR to OLR; the ORANGE II PLUS trial is a multi-institutional study involving 10 centers in Europe and estimated to be completed in October 2016 [33]. The Oslo-CoMet study in Norway is estimated to be completed in December 2015 [34]. Currently, all the available data are based on case-control studies, case series, and meta-analyses [16••, 35].
A recent meta-analysis of 610 patients compared laparoscopic versus open liver resection for metastatic colorectal cancer. It included eight retrospective studies case matched for demographics, tumor characteristics, and operative interventions and compared 242 LLRs to 368 OLRs. Compared to the OLR group, the LLR group had lower estimated blood loss (EBL, 385 vs 263 ml), transfusion rate (19.8 vs 9.9 %), length of stay (8.8 vs 6.5 days), and overall complication rate (20.3 vs 33.2 %). There was no difference in operative time, margins positivity, liver-specific complications, or 30-day mortality. Oncologically, there was no difference in 1-, 2-, and 5-year disease-free survival or overall survival between the groups [16••]. Of note, the median number of tumors was 1.4 and 1.5 for the LLR and OLR groups, respectively. Since there is no more restriction on the number of colorectal cancer liver metastasectomy when possible, the available data is only true for patients with limited metastasis. Other meta-analyses showed similar results with regard to EBL, transfusion rate, overall complications, and long-term oncological outcomes [36–38]. On the other hand, they showed lower R1 resection with LLR and this was attributed to the routine use of intraop US and the tendency to perform OLR when the lesions are close to the hilum [36–38]. Moreover, an initial laparoscopic approach for CRCLM allowed a higher chance of subsequent hepatectomies for liver recurrence compared to OLR. This is possibly attributed to fewer adhesions and less tissue damage conferred by the laparoscopic approach [39]. In addition, patients undergoing minimally invasive surgery for CRCLM get earlier adjuvant chemotherapy compared to OLR [40]. Laparoscopy offers not only better clinical outcomes, but it is also more cost-efficient compared to OLR. Despite the higher upfront OR cost, a shorter length of stay after LLR is a possible explanation [41, 42].
LLR for CRCLM is safe and feasible. It decreases transfusion rate, length of stay, and overall morbidities without compromising short- and long-term oncological outcomes. While all these advantages are promising in liver surgery, it is noteworthy to mention that the available data is based on retrospective studies that pose a selection bias. Therefore, a careful selection of patients and a high experience in minimally invasive surgery are warranted if laparoscopy is used in CRCLM resection.
Robotic Liver Resection
Background
The first robotic-assisted surgery was performed in 1983 in Vancouver. Since then, the field of robotics has evolved and advanced. In 2000, the FDA approved the use of the da Vinci for general surgery in the USA [43]. Shortly thereafter, in 2001, the first robotic liver surgery was performed by Giulianotti in Italy [44]. Since then, robotic liver resection (RLR) has been slowly adopted as it is believed to overcome some of the limitations of laparoscopy. The robot provides better ergonomics with 7 degrees of freedom compared to 4 in laparoscopy. In addition, it eliminates the fulcrum effect caused by rigid laparoscopic instruments. This allows the ability to mimic human hand dexterity and facilitates easier tissue handling, precise suturing, operating in small spaces, and working at angles that are not possible in laparoscopy. This is very attractive in hepatic surgery due to its complexity, especially in resecting lesions in the posterior-superior segments (VII and VIII). Tumors in these locations require curved transections, which are very challenging with laparoscopy while the robotic arms can overcome these difficulties [45]. Moreover, the robotic platform improves visual perception by providing three-dimensional and magnified images of the operative field. In combination with the ability to eliminate hand tremor, the robot allows more precise and delicate movements. The surgeon is also able to control the camera and retractors and lock them into position, eliminating inappropriate camera control or retractions. Finally, the presence of computerized console can allow image-guided surgery which is becoming attractive in hepatobiliary surgery [46]. Despite these advantages, RLR is still not widely used and is considered a novel technique still under investigation [24].
Technique
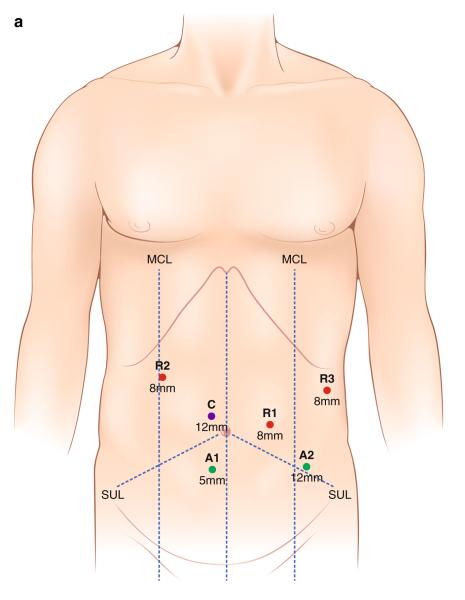
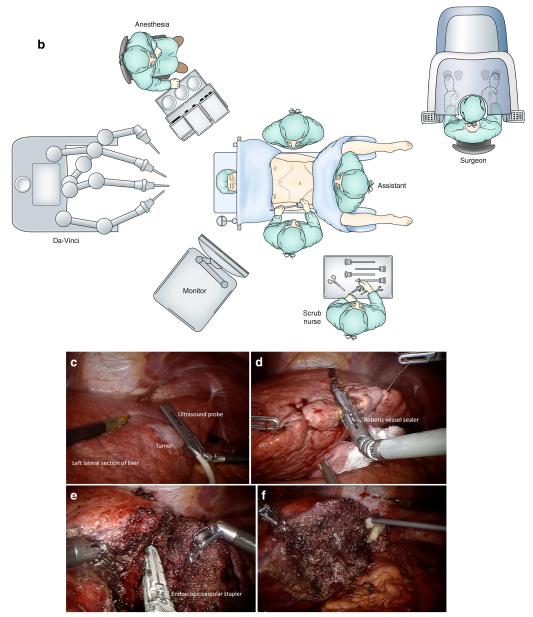
The indications for RLR are similar to those for LLR with the emphasis that this is a new technique that should be used by a surgeon experienced in hepatobiliary and laparoscopic surgery. On the other hand, RLR is contraindicated in patients with marginal cardiopulmonary reserve, poor pneumoperitoneum tolerance, malignant hepatic lesions invading major vessels, extensive subcapsular involvement, or the need of vascular reconstruction. Robotic liver resection technique varies according to surgeons’ preference, location of the mass, and the segment/section to be resected. A patient is positioned in a supine reverse Trendelenburg position with the right side up for right or posterior liver lesions. A split-leg table is recommended for the assistant surgeon to stand between the patient’s legs. The authors’ trocar placement for a left lateral sectionectomy is depicted in Fig. 1. The da Vinci Si II System (Intuitive Surgical Inc., Sunnyvale, CA) requires three 8-mm incisions and one 12-mm trocar for robotic instruments and camera placement, respectively. Additional 5- and 12-mm trocars are placed in the lower abdomen for the assistant surgeon. After mapping out the lesion and vascular anatomy with intraoperative ultrasound, traction stitches on the specimen side are placed. Hepatic transection is achieved with a combination of cautery, robotic vessel sealer, and a robotic bipolar dissector. Endoscopic vascular staplers are used for larger vascular or biliary pedicles. Laparoscopic bipolar energy with the Aquamantys Endo DBS 8.7 (Medtronic, Minneapolis, MN) is commonly used by the assistant surgeon at bedside for final hemostasis. Drain placement should be considered if there is a concern for biliary leak, biloma formation, or bleeding. The specimen is extracted in an endoscopic bag by extending the 12-mm trocar utility port incision. All trocars or incisions larger than 12 mm are closed with a Vicryl 0 suture. The authors’ technique and main steps for robotic left lateral sectionectomy are summarized in Fig. 1.
Fig. 1.
a Trocar placement for robotic left lateral sectionectomy (red (R1–3), 8-mm trocars for robotic arms; purple (C) 12 mm for camera, and green (A1–2) 5- and 12-mm assistant ports). MCL is the midclavicular line, SUL the spinoumbilical line. b OR setup for robotic left lateral sectionectomy. c Robotic intraoperative ultrasound of the liver defining tumor extension and vascular/biliary anatomy. d Transection of the liver parenchyma with a robotic vessel sealer. e Dissection and transection of major vascular pedicles with an endoscopic vascular stapler. f Final hemostasis of the transected liver surface. A specimen is extracted in an endoscopic bag through an extended utility port (not shown)
Outcomes
The current literature on RLR is limited to small case series and retrospective comparative studies with LLR. Most studies include the resection of both benign and malignant lesions including hepatocellular carcinoma (HCC) and CRC metastasis. In addition, there is a lack of long-term oncological follow-up as this technique has been recently adopted. Moreover, there are no available comparative reports of the short- and long-term oncologic outcomes following RLR for CRC metastasis alone [45, 47–63].
Giulianotti et al. published the largest series on RLR in 2011 [61]. The series included 70 patients with 42 having malignant tumors and 16 having CRC metastasis. Twenty-seven patients underwent major RLR including 20 right hepatectomy, 5 left hepatectomy, and 2 right trisectionectomy cases. The median operative time was 198 min (90–459 min) for minor RLR and 313 min (220–480 min) for major ones. The conversion rate was 5.7 %, EBL was 262 cm3 (20–2000 cm3), and morbidity was 21.4 % with 12.9 % being major.
Tsung et al. published the largest comparative study between RLR and LLR where 57 robotic liver resections were matched 2:1 to laparoscopic liver resections [47]. Twenty-one of the patients had CRCLM. RLR had longer operative time (253 vs 198.5 min). On the other hand, RLR had similar conversion rate (7 vs 8.8 %), total complication rate (19 vs 26 %), and margin status compared to LLR.
A meta-analysis of seven studies comparing 479 LLRs with 215 RLRs showed that EBL and operative time were higher in RLR, while the conversion rate, R1 resection rate, hospital stay, mortality, and morbidity were similar to LLR [64]. Another meta-analysis of nine comparative trials comparing 254 RLRs and 522 LLRs found similar results. The operative time was longer in RLR, but there was no difference in conversion rate, negative margins, morbidity, or mortality. EBL was higher in RLR, but the difference was not statistically significant [65••].
The longer operative time in RLR could be explained by the added time of docking the robotic arms, slower hepatic parenchyma transection, and early stage of the surgeons’ learning curve. Tsung et al. and Montalti showed that the operative time in RLR is lower in later cases compared to early cases (381 vs 232 min) [47, 64].
Postoperative clinical outcomes were similar between both techniques. The 30-day mortality rate was null in a meta-analysis for both RLR and LLR. Postoperative morbidity was similar with bile leak and intra-abdominal collection as the main reasons for complications. The length of stay was not different, but the cost of robotic surgery was higher [62].
Finally, the oncological outcomes were equivalent with similar R1 resection rates. No port site recurrences were evident, although there are no good data on long-term outcomes and an interpretation of the effect of RLR on local recurrence rate and disease-free survival is not possible [66].
In conclusion, RLR is safe and feasible in selected patients and in the hands of an experienced surgeon in both open liver surgery and minimally invasive surgery. Perioperative outcomes are similar with RLR having a higher operative time and cost. While pathological outcomes are similar, there is a need of long-term follow-up to determine oncological outcomes with respect to recurrence and survival. There might be an edge to RLR over LLR as it allows more complex hepatectomies in some studies [65••]. High-quality studies are needed to draw conclusions on the short- and long-term outcomes of this technique. Currently, it is considered a promising new tool and should require an institutional ethical approval as well as a reporting registry for these cases [24].
Laparoscopic Ablative Techniques
The liver is the main site of metastasis of colorectal cancer. It is the only site of distant disease in 40 % of patients and is present in 80 % of stage IV patients [2–4]. The majority of patients with liver metastasis have unresectable disease; in fact, less than 25 % of patients present with curable disease [67–69]. While patients with unresectable disease have dismal 5-year survival, patients who undergo curative resection have a 5-year survival rate as high as 40 % [68]. With the advancement of surgical techniques and post-op care, the criteria for resectability have expanded. It is safe to resect up to 80 % of healthy liver tissue with a mortality less than 5 % in large surgical centers [68, 70]. Patients with bulky, bilobar disease or poor hepatic reserve after resection are considered inoperable. Alternative ablative techniques can be used to complement or replace surgical resection when not possible. Among these therapies, we will discuss the use of RFA and MWC. While these procedures can be performed open, laparoscopically, and percutaneously (CT or ultrasound-guided), we will describe them in the setting of minimally invasive surgery.
Laparoscopic Radio-frequency Ablation
In 1990, McGahan et al. described the ablation of liver lesions using a monopolar radio-frequency electrocautery [71]. The passage of an alternating current through the liver parenchyma will generate heat leading to tissue necrosis and protein coagulation [72]. Temperature is allowed to reach 90–100 °C since a tip temperature higher than 110 °C causes tissue desiccation that acts as an insulator and thus decreases the efficiency of the ablation. In order to avoid this problem and to increase the area of ablated liver tissue, the RFA probe was modified. Electrode cooling and several array needles have been introduced [69, 72]. In addition, RFA is less effective when the target lesion is next to large blood vessels as the heat is dissipated by the cooler blood flow, a phenomenon called the heat-sink effect. Initially, RFA was used as a palliative measure in conjunction with chemotherapy to treat nonsurgical patients with colorectal cancer liver metastasis.
Siperstein et al. described one of the largest series of laparoscopic RFA for unresectable metastatic colorectal cancer. It involved 234 consecutive patients who were deemed nonsurgical either due to a disease burden or due to a prohibitive surgical risk. Inclusion criteria included nonresponders to chemotherapy (80 % of patients), the presence of extrahepatic disease (23.5 % of patients), and up to 12 lesions with the largest up to 10 cm. The average number of lesions and size were 2.8 ± 0.14 and 3.9 ± 0.2 cm, respectively. The median lag time between diagnosis and RFA was 8 months, explained by a trial of medical therapy (mainly chemotherapy). The median survival was 32 months since the diagnosis of the metastatic disease and was worse with a greater number of lesions (>3) and higher CEA (>200 ng/ml) [73]. A randomized controlled trial comparing RFA to RFA with a systemic treatment in nonresectable colorectal cancer liver metastasis showed similar findings. There was no difference in 30-month overall survival, but the 3-year progression-free survival was higher in the RFA group (27.6 vs 10.6 %) [74].
The main controversy is the use of RFA for solitary and resectable lesions instead of surgery since it is less invasive and is associated with fewer comorbidities.
Aliyev et al. described the oncological outcomes of laparoscopic RFA compared to resection of solitary CRCLM ≤3 cm. RFA patients had higher ASA score, more cardiopulmonary comorbidities, and deeper tumors. Both groups had similar age, gender, CEA level, tumor size, and synchronous versus metachronous disease. The local recurrence for RFA was higher than the resection groups (18 vs 4 %). On the other hand, there was no statistical difference in overall cancer-specific 5-year survival (47 vs 57 %) or median disease-free survival (25 vs 22 months). The author concluded that RFA is acceptable for patients with high surgical risk for a formal liver resection, keeping in mind the higher local recurrence [75].
In a meta-analysis of 95 studies including 5224 treated liver tumors, of which 14.7 % were CRCLM, local recurrence was found to be higher in lesions larger than 3 cm and with a percutaneous approach [76•]. One advantage of laparoscopy is the ability to explore the abdomen and to use intraop US which increases sensitivity compared to CT scan for detecting liver tumors. This can explain the superiority of laparoscopic RFA over a percutaneous approach [77].
A recent meta-analysis, including 10 studies comparing radio-frequency ablation to liver resection, has shown worse overall survival and disease-free survival and concluded that RFA should only be used in patients who are not fit for surgical resection.
In conclusion, surgical resection should be the standard of care in patients with resectable CRCLM. RFA can be used for inoperable cases or as an adjunct to liver resection. In patients who have resectable disease and cannot tolerate a hepatectomy due to comorbidities or poor hepatic reserve, RFA can be used for small lesions knowing that local recurrence is higher, and mandating a close follow-up.
Laparoscopic Microwave Coagulation
In 1979, Tabuse reported using microwave coagulation (MWC) as a hemostatic technique to minimize bleeding during hepatic resections [78]. Since then, this technology has evolved and been used to ablate primary and secondary liver lesions. Most of the literature describes the use of MWC in HCC until recently where it has been used to ablate CRCLM [79–86].
Microwave coagulation generates an electromagnetic radiation of high frequency (900 MHz to 2.45 GHz), causing water molecule oscillation. This will result in frictional heating and, subsequently, coagulation necrosis of the hepatic parenchyma. Microwave coagulation relies on active heating in comparison to RFA passive heating, minimizing the concern of tissue desiccation and the heat-sink effect. In addition, it creates wider ablation zones in a shorter time interval than RFA which might confer therapeutic advantages [82, 87–89]. In fact, a retrospective matched cohort study of CRCLM patients undergoing MWC versus RFA showed that the ablation site recurrence at 2 years was lower for lesions ablated by MWC (7 vs 18 %) [84].
Shibata et al. reported the first clinical trial comparing open MWC to hepatic resection in patients with resectable CRCLM. There was no difference in the median survival between the MWC group (27 months) and the surgical group (25 months) [81]. While the initial approach was mainly open or percutaneous, laparoscopic MWC has gained more attention. The feasibility and safety of laparoscopic MWC was shown in a retrospective study including 57 patients with liver tumors. Forty-six patients had secondary tumors, mainly CRCLM. There were no intraop complications, while 4 patients developed hepatic abscesses, two of which required drainage. Follow-up imaging showed complete necrosis of the tumors, and the mean overall survival was 22.6 months [80, 90•]. There is a paucity of studies describing the long-term outcomes following laparoscopic MWC for CRCLM. A recent report described the oncological outcomes following open MWC for liver tumors. It included 416 tumors of which 81 % were CRCLM. The overall survival at 4 years was 58.3 % for CRCLM. Local recurrence was 7.9 % with a median follow-up of 20.5 months. Recurrence rates increased with tumor size and were 1 % for 1-cm tumors, 9.3 % for 1–3-cm tumors, and 33 % for tumors larger than 3 cm [85]. While MWC seems to offer a favorable local control for small lesions, there is a need for better comparative studies. It can be used as an alternative modality to surgery in order to achieve a cure in nonsurgical patients.
Conclusions
Minimally invasive liver surgery for CRCLM has evolved significantly over the last two decades. Laparoscopy has become the standard of care for minor liver resections. Until more data is available from currently undertaken randomized trials, laparoscopic major liver resection should be performed by an experienced surgeon in liver and minimally invasive surgery. Robotic surgery for liver resections is a promising novel technique and may overcome the limitations of laparoscopy. Further data is needed to define benefits, indications, and long-term outcomes of RLR. While surgery is the standard of care for resectable CRCLM, as it confers the best chance of cure, adjuncts can be used in case of high surgical risks or in inoperable patients. Laparoscopic RFA and MWC are additional tools to achieve a local control of lesions nonamenable to surgical resection.
Acknowledgment
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award Number UL1TR001105. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Ibrahim Nassour and Patricio M. Polanco declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Paper of particular interests, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Frankel TL, D’Angelica MI. Hepatic resection for colorectal metastases. J Surg Oncol. 2013;109(1):2–7. doi: 10.1002/jso.23371. doi: 10.1002/jso.23371. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Ward EM, Jemal A. Trends in colorectal cancer incidence rates in the United States by tumor location and stage, 1992-2008. Cancer Epidemiol Biomarkers Prev. 2012;21(3):411–6. doi: 10.1158/1055-9965.EPI-11-1020. doi: 10.1158/1055-9965.EPI-11-1020. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Nordlinger B, Adam R, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42(14):2212–21. doi: 10.1016/j.ejca.2006.04.012. doi: 10.1016/j.ejca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. Advanced Colorectal Cancer Meta-Analysis Project. J Clin Oncol. 1992;10(6):896–903. doi: 10.1200/JCO.1992.10.6.896. [No author] [DOI] [PubMed] [Google Scholar]
- 6.Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343(13):905–14. doi: 10.1056/NEJM200009283431302. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 7.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–7. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22(1):23–30. doi: 10.1200/JCO.2004.09.046. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham D, Pyrhönen S, James RD, et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet. 1998;352(9138):1413–8. doi: 10.1016/S0140-6736(98)02309-5. doi: 10.1016/S0140-6736(98)02309-5. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42. doi: 10.1056/NEJMoa032691. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 11.Nathan H, de Jong MC, Pulitano C, et al. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. J Am Coll Surg. 2010;210(5):755–66. doi: 10.1016/j.jamcollsurg.2009.12.041. doi: 10.1016/j.jamcollsurg.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 12.Hamady ZZR, Lodge JPA, Welsh FK, et al. One-millimeter cancer-free margin is curative for colorectal liver metastases: a propensity score case-match approach. Ann Surg. 2014;259(3):543–8. doi: 10.1097/SLA.0b013e3182902b6e. doi: 10.1097/SLA.0b013e3182902b6e. [DOI] [PubMed] [Google Scholar]
- 13.Mavros MN, de Jong M, Dogeas E, Hyder O, Pawlik TM. Impact of complications on long-term survival after resection of colorectal liver metastases. Br J Surg. 2013;100(5):711–8. doi: 10.1002/bjs.9060. doi: 10.1002/bjs.9060. [DOI] [PubMed] [Google Scholar]
- 14.Andreou A, Aloia TA, Brouquet A, et al. Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann Surg. 2013;257(6):1079–88. doi: 10.1097/SLA.0b013e318283a4d1. doi: 10.1097/SLA.0b013e318283a4d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beppu T, Sakamoto Y, Hasegawa K, et al. A nomogram predicting disease-free survival in patients with colorectal liver metastases treated with hepatic resection: multicenter data collection as a project study for hepatic surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2012;19(1):72–84. doi: 10.1007/s00534-011-0460-z. doi: 10.1007/s00534-011-0460-z. [DOI] [PubMed] [Google Scholar]
- 16••.Schiffman SC, Kim KH, Tsung A, Marsh JW, Geller DA. Laparoscopic versus open liver resection for metastatic colorectal cancer: a metaanalysis of 610 patients. Surgery. 2015;157(2):211–22. doi: 10.1016/j.surg.2014.08.036. doi: 10.1016/j.surg.2014.08.036. This meta-analysis shows that LLR has better perioperative outcomes without compromising oncological outcomes compared to OLR.
- 17.Hasegawa Y, Nitta H, Sasaki A, et al. Long-term outcomes of laparoscopic versus open liver resection for liver metastases from colorectal cancer: a comparative analysis of 168 consecutive cases at a single center. Surgery. 2015;157(6):1065–72. doi: 10.1016/j.surg.2015.01.017. doi: 10.1016/j.surg.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13(1):51–64. doi: 10.1634/theoncologist.2007-0142. doi: 10.1634/theoncologist.2007-0142. [DOI] [PubMed] [Google Scholar]
- 19.Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg. 2007;11(8):1057–77. doi: 10.1007/s11605-006-0061-3. doi: 10.1007/s11605-006-0061-3. [DOI] [PubMed] [Google Scholar]
- 20.Gagner M, Rheault M, Dubuc J. Laparoscopic partial hepatectomy for liver tumor. Surg Endosc. 1992;6:97–8. [Google Scholar]
- 21.Azagra JS, Goergen M, Gilbart E, Jacobs D. Laparoscopic anatomical (hepatic) left lateral segmentectomy-technical aspects. Surg Endosc. 1996;10(7):758–61. doi: 10.1007/BF00193052. [DOI] [PubMed] [Google Scholar]
- 22.Talamini MA. Advanced therapy in minimally invasive surgery. PMPH; USA: 2006. [Google Scholar]
- 23.Wayand W, Woisetschläger R. Laparoscopic resection of liver metastasis. Chirurg. 1993;64(3):195–7. [PubMed] [Google Scholar]
- 24.Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619–29. doi: 10.1097/SLA.0000000000001184. doi: 10.1097/SLA.0000000000001180. [DOI] [PubMed] [Google Scholar]
- 25.Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg. 2009;250:825–30. doi: 10.1097/sla.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 26.Sahay SJ, Fazio F, Cetta F, Chouial H, Lykoudis PM, Fusai G. Laparoscopic left lateral hepatectomy for colorectal metastasis is the standard of care. J BUON. 2015;20(4):1048–53. [PubMed] [Google Scholar]
- 27.Cherqui D, Husson E, Hammoud R, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000;232(6):753–62. doi: 10.1097/00000658-200012000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geller DA, Tsung A. Long-term outcomes and safety of laparoscopic liver resection surgery for hepatocellular carcinoma and metastatic colorectal cancer. J Hepatobiliary Pancreat Sci. 2015;22(10):728–30. doi: 10.1002/jhbp.278. doi: 10.1002/jhbp.278. [DOI] [PubMed] [Google Scholar]
- 29.Tamandl D, Herberger B, Gruenberger B, et al. Adequate preoperative staging rarely leads to a change of intraoperative strategy in patients undergoing surgery for colorectal cancer liver metastases. Surgery. 2008;143(5):648–57. doi: 10.1016/j.surg.2007.11.020. doi: 10.1016/j.surg.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 30.van Vledder MG, Pawlik TM, Munireddy S, Hamper U, de Jong MC, Choti MA. Factors determining the sensitivity of intraoperative ultrasonography in detecting colorectal liver metastases in the modern era. Ann Surg Oncol. 2010;17(10):2756–63. doi: 10.1245/s10434-010-1108-y. doi: 10.1245/s10434-010-1108-y. [DOI] [PubMed] [Google Scholar]
- 31.Conlon R, Jacobs M, Dasgupta D, Lodge JPA. The value of intra-operative ultrasound during hepatic resection compared with improved preoperative magnetic resonance imaging. Eur J Ultrasound. 2003;16(3):211–6. doi: 10.1016/s0929-8266(02)00075-7. [DOI] [PubMed] [Google Scholar]
- 32.Viganò L, Ferrero A, Amisano M, Russolillo N, Capussotti L. Comparison of laparoscopic and open intraoperative ultrasonography for staging liver tumours. Br J Surg. 2013;100(4):535–42. doi: 10.1002/bjs.9025. doi: 10.1002/bjs.9025. [DOI] [PubMed] [Google Scholar]
- 33.The ORANGE II PLUS [Accessed 15 Dec 2015];Trial: open versus laparoscopic hemihepatectomy. 2015 https://clinicaltrials.gov/ct2/show/NCT01441856.
- 34. [Accessed 15 Dec 2015];Oslo Randomized Laparoscopic Versus Open Liver Resection for Colorectal Metastases Study (Oslo-CoMet) 2015 https://clinicaltrials.gov/ct2/show/NCT01516710?term=oslo+comet+study&rank=1.
- 35.Parks KR, Kuo Y-H, Davis JM, O’Brien B, Hagopian EJ. Laparoscopic versus open liver resection: a meta-analysis of long-term outcome. HPB. 2013;16(2):109–18. doi: 10.1111/hpb.12117. doi: 10.1111/hpb.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo L-X, Yu Z-Y, Bai Y-N. Laparoscopic hepatectomy for liver metastases from colorectal cancer: a meta-analysis. J Laparoendosc Adv Surg Tech A. 2014;24(4):213–22. doi: 10.1089/lap.2013.0399. doi: 10.1089/lap.2013.0399. [DOI] [PubMed] [Google Scholar]
- 37.Wei M, He Y, Wang J, Chen N, Zhou Z, Wang Z. Laparoscopic versus open hepatectomy with or without synchronous colectomy for colorectal liver metastasis: a meta-analysis. PLoS One. 2014;9(1):e87461–9. doi: 10.1371/journal.pone.0087461. doi: 10.1371/journal.pone.0087461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, Xiao Y, Wu L, Li B, Li H. Laparoscopic liver resection as a safe and efficacious alternative to open resection for colorectal liver metastasis: a meta-analysis. BMC Surg. 2013;13:44. doi: 10.1186/1471-2482-13-44. doi: 10.1186/1471-2482-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montalti R, Berardi G, Laurent S, et al. Laparoscopic liver resection compared to open approach in patients with colorectal liver metastases improves further resectability: oncological outcomes of a case-control matched-pairs analysis. Eur J Surg Oncol. 2014;40(5):536–44. doi: 10.1016/j.ejso.2014.01.005. doi: 10.1016/j.ejso.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Tohme S, Goswami J, Han K, et al. Minimally invasive resection of colorectal cancer liver metastases leads to an earlier initiation of chemotherapy compared to open surgery. J Gastrointest Surg. 2015 doi: 10.1007/s11605-015-2962-5. doi: 10.1007/s11605-015-2962-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cannon RM, Scoggins CR, Callender GG, Quillo A, McMasters KM, Martin RCG. Financial comparison of laparoscopic versus open hepatic resection using deviation-based cost modeling. Ann Surg Oncol. 2013;20(9):2887–92. doi: 10.1245/s10434-013-2993-7. doi: 10.1245/s10434-013-2993-7. [DOI] [PubMed] [Google Scholar]
- 42.Vanounou T, Steel JL, Nguyen KT, et al. Comparing the clinical and economic impact of laparoscopic versus open liver resection. Ann Surg Oncol. 2009;17(4):998–1009. doi: 10.1245/s10434-009-0839-0. doi: 10.1245/s10434-009-0839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. [Accessed 15 Dec 2015];History of surgical robotics. 2015 http://www.avrasurgicalrobotics.com/historyofsurgicalrobotics.html.
- 44.Giulianotti PC. Robotics in general surgery. Arch Surg. 2003;138(7):777. doi: 10.1001/archsurg.138.7.777. doi: 10.1001/archsurg.138.7.777. [DOI] [PubMed] [Google Scholar]
- 45.Casciola L, Patriti A, Ceccarelli G, Bartoli A, Ceribelli C, Spaziani A. Robot-assisted parenchymal-sparing liver surgery including lesions located in the posterosuperior segments. Surg Endosc. 2011;25(12):3815–24. doi: 10.1007/s00464-011-1796-9. doi: 10.1007/s00464-011-1796-9. [DOI] [PubMed] [Google Scholar]
- 46.Kingham TP, Scherer MA, Neese BW, Clements LW, Stefansic JD, Jarnagin WR. Image-guided liver surgery: intraoperative projection of computed tomography images utilizing tracked ultrasound. HPB. 2012;14(9):594–603. doi: 10.1111/j.1477-2574.2012.00487.x. doi: 10.1111/j.1477-2574.2012.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsung A, Geller DA, Sukato DC, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg. 2014;259(3):549–55. doi: 10.1097/SLA.0000000000000250. doi: 10.1097/SLA.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 48.Berber E, Akyildiz HY, Aucejo F, Gunasekaran G, Chalikonda S, Fung J. Robotic versus laparoscopic resection of liver tumours. HPB. 2010;12(8):583–6. doi: 10.1111/j.1477-2574.2010.00234.x. doi: 10.1111/j.1477-2574.2010.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji W-B, Wang H-G, Zhao Z-M, Duan W-D, Lu F, Dong J-H. Robotic-assisted laparoscopic anatomic hepatectomy in China: initial experience. Ann Surg. 2011;253(2):342–8. doi: 10.1097/SLA.0b013e3181ff4601. doi: 10.1097/SLA.0b013e3181ff4601. [DOI] [PubMed] [Google Scholar]
- 50.Tranchart H, Ceribelli C, Ferretti S, Dagher I, Patriti A. Traditional versus robot-assisted full laparoscopic liver resection: a matched-pair comparative study. World J Surg. 2014;38(11):2904–9. doi: 10.1007/s00268-014-2679-8. doi: 10.1007/s00268-014-2679-8. [DOI] [PubMed] [Google Scholar]
- 51.Wu Y-M, Hu R-H, Lai H-S, Lee P-H. Robotic-assisted minimally invasive liver resection. Asian J Surg. 2014;37(2):53–7. doi: 10.1016/j.asjsur.2014.01.015. doi: 10.1016/j.asjsur.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 52.Spampinato MG, Coratti A, Bianco L, et al. Perioperative outcomes of laparoscopic and robot-assisted major hepatectomies: an Italian multi-institutional comparative study. Surg Endosc. 2014;28(10):2973–9. doi: 10.1007/s00464-014-3560-4. doi: 10.1007/s00464-014-3560-4. [DOI] [PubMed] [Google Scholar]
- 53.Choi GH, Choi SH, Kim SH, et al. Robotic liver resection: technique and results of 30 consecutive procedures. Surg Endosc. 2012;26(8):2247–58. doi: 10.1007/s00464-012-2168-9. doi: 10.1007/s00464-012-2168-9. [DOI] [PubMed] [Google Scholar]
- 54.Chan OCY, Tang C-N, Lai ECH, Yang GPC, Li MKW. Robotic hepatobiliary and pancreatic surgery: a cohort study. J Hepatobiliary Pancreat Sci. 2011;18(4):471–80. doi: 10.1007/s00534-011-0389-2. doi: 10.1007/s00534-011-0389-2. [DOI] [PubMed] [Google Scholar]
- 55.Lai ECH, Tang C-N, Li MKW. Robot-assisted laparoscopic hemihepatectomy: technique and surgical outcomes. Int J Surg. 2012;10(1):11–5. doi: 10.1016/j.ijsu.2011.10.005. doi: 10.1016/j.ijsu.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Patriti A, Ceccarelli G, Bartoli A, Spaziani A, Lapalorcia LM, Casciola L. Laparoscopic and robot-assisted one-stage resection of colorectal cancer with synchronous liver metastases: a pilot study. J Hepatobiliary Pancreat Surg. 2009;16(4):450–7. doi: 10.1007/s00534-009-0073-y. doi: 10.1007/s00534-009-0073-y. [DOI] [PubMed] [Google Scholar]
- 57.Wakabayashi G, Sasaki A, Nishizuka S, Furukawa T, Kitajima M. Our initial experience with robotic hepato-biliary-pancreatic surgery. J Hepatobiliary Pancreat Sci. 2011;18(4):481–7. doi: 10.1007/s00534-011-0388-3. doi: 10.1007/s00534-011-0388-3. [DOI] [PubMed] [Google Scholar]
- 58.Croner RS, Perrakis A, Brunner M, Matzel KE, Hohenberger W. Pioneering robotic liver surgery in Germany: first experiences with liver malignancies. Front Surg. 2015;2:18. doi: 10.3389/fsurg.2015.00018. doi: 10.3389/fsurg.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Felli E, Santoro R, Colasanti M, Vennarecci G, Lepiane P, Ettorre GM. Robotic liver surgery: preliminary experience in a tertiary hepato-biliary unit. Updates Surg. 2015;67(1):27–32. doi: 10.1007/s13304-015-0285-4. doi: 10.1007/s13304-015-0285-4. [DOI] [PubMed] [Google Scholar]
- 60.Montalti R, Patriti A, Troisi RI. Robotic versus laparoscopic hepatectomy. Ann Surg. 2015;262(2):e70–2. doi: 10.1097/SLA.0000000000000701. doi: 10.1097/SLA.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 61.Giulianotti PC, Coratti A, Sbrana F, et al. Robotic liver surgery: results for 70 resections. Surgery. 2011;149(1):29–39. doi: 10.1016/j.surg.2010.04.002. doi: 10.1016/j.surg.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 62.Yu Y-D, Kim K-H, Jung D-H, et al. Robotic versus laparoscopic liver resection: a comparative study from a single center. Langenbecks Arch Surg. 2014;399(8):1039–45. doi: 10.1007/s00423-014-1238-y. doi: 10.1007/s00423-014-1238-y. [DOI] [PubMed] [Google Scholar]
- 63.Packiam V, Bartlett DL, Tohme S, et al. Minimally invasive liver resection: robotic versus laparoscopic left lateral sectionectomy. J Gastrointest Surg. 2012;16(12):2233–8. doi: 10.1007/s11605-012-2040-1. doi: 10.1007/s11605-012-2040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Montalti R. Outcomes of robotic vs laparoscopic hepatectomy: a systematic review and meta-analysis. WJG. 2015;21(27):8441–12. doi: 10.3748/wjg.v21.i27.8441. doi: 10.3748/wjg.v21.i27.8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65••.Qiu J, Chen S, Chengyou D. A systematic review of robotic-assisted liver resection and meta-analysis of robotic versus laparoscopic hepatectomy for hepatic neoplasms. Surg Endosc. 2015 doi: 10.1007/s00464-015-4306-7. doi: 10.1007/s00464-015-4306-7.. This is the largest meta-analysis showing that operative time was longer in RLR, but there was no difference in conversion rate, negative margins, morbidity, or mortality compared to LLR.
- 66.Ocuin LM, Tsung A. Robotic liver resection for malignancy: current status, oncologic outcomes, comparison to laparoscopy, and future applications. J Surg Oncol. 2015;112(3):295–301. doi: 10.1002/jso.23901. doi: 10.1002/jso.23901. [DOI] [PubMed] [Google Scholar]
- 67.Abdalla EK, Vauthey J-N, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239(6):818–27. doi: 10.1097/01.sla.0000128305.90650.71. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pwint TP, Midgley R, Kerr DJ. Regional hepatic chemotherapies in the treatment of colorectal cancer metastases to the liver. Semin Oncol. 2010;37(2):149–59. doi: 10.1053/j.seminoncol.2010.03.005. doi: 10.1053/j.seminoncol.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 69.Rocha FG, D’Angelica M. Treatment of liver colorectal metastases: role of laparoscopy, radiofrequency ablation, and microwave coagulation. J Surg Oncol. 2010;102(8):968–74. doi: 10.1002/jso.21720. doi: 10.1002/jso.21720. [DOI] [PubMed] [Google Scholar]
- 70.Morris-Stiff G, Marangoni G, Hakeem A, et al. Redefining major hepatic resection for colorectal liver metastases: analysis of 1111 liver resections. Int J Surg. 2015 doi: 10.1016/j.ijsu.2015.07.711. doi: 10.1016/j.ijsu.2015.07.711. [DOI] [PubMed] [Google Scholar]
- 71.McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Investig Radiol. 1990;25(3):267. doi: 10.1097/00004424-199003000-00011. [DOI] [PubMed] [Google Scholar]
- 72.Decadt B, Siriwardena AK. Radiofrequency ablation of liver tumours: systematic review. Lancet Oncol. 2004;5(9):550–60. doi: 10.1016/S1470-2045(04)01567-0. doi: 10.1016/S1470-2045(04)01567-0. [DOI] [PubMed] [Google Scholar]
- 73.Siperstein AE, Berber E, Ballem N, Parikh RT. Survival after radio-frequency ablation of colorectal liver metastases: 10-year experience. Ann Surg. 2007;246(4):559–65. doi: 10.1097/SLA.0b013e318155a7b6. doi: 10.1097/SLA.0b013e318155a7b6. Discussion 565–7. [DOI] [PubMed] [Google Scholar]
- 74.Ruers T, Punt C, Van Coevorden F, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004) Ann Oncol. 2012;23(10):2619–26. doi: 10.1093/annonc/mds053. doi: 10.1093/annonc/mds053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aliyev S, Agcaoglu O, Aksoy E, et al. Efficacy of laparoscopic radiofrequency ablation for the treatment of patients with small solitary colorectal liver metastasis. Surgery. 2013;154(3):556–62. doi: 10.1016/j.surg.2013.03.009. doi: 10.1016/j.surg.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 76•.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242(2):158–71. doi: 10.1097/01.sla.0000171032.99149.fe. doi: 10.1097/01.sla.0000171032.99149.fe. This meta-analysis shows that a tumor bigger than 3 cm has a higher recurrence rate if treated by RFA.
- 77.Foroutani A. Laparoscopic ultrasound vs triphasic computed tomography for detecting liver tumors. Arch Surg. 2000;135(8):933–8. doi: 10.1001/archsurg.135.8.933. doi: 10.1001/archsurg.135.8.933. [DOI] [PubMed] [Google Scholar]
- 78.Tabuse K. A new operative procedure of hepatic surgery using a microwave tissue coagulator. Nihon Geka Hokan. 1979;48(2):160–72. [PubMed] [Google Scholar]
- 79.Yokoyama T, Egami K, Miyamoto M, et al. Percutaneous and laparoscopic approaches of radiofrequency ablation treatment for liver cancer. J Hepatobiliary Pancreat Surg. 2003;10(6):425–7. doi: 10.1007/s00534-002-0830-7. doi: 10.1007/s00534-002-0830-7. [DOI] [PubMed] [Google Scholar]
- 80.Jagad RB, Koshariya M, Kawamoto J, et al. Laparoscopic micro-wave ablation of liver tumors: our experience. Hepatogastroenterology. 2008;55(81):27–32. [PubMed] [Google Scholar]
- 81.Shibata T, Niinobu T, Ogata N, Takami M. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer. 2000;89(2):276–84. [PubMed] [Google Scholar]
- 82.Jiao LR, Habib NA. Experimental study of large-volume microwave ablation in the liver. Br J Surg. 2003;90(1):122. doi: 10.1002/bjs.4088. doi: 10.1002/bjs.4088. [DOI] [PubMed] [Google Scholar]
- 83.Seki T, Wakabayashi M, Nakagawa T, et al. Percutaneous micro-wave coagulation therapy for solitary metastatic liver tumors from colorectal cancer: a pilot clinical study. Am J Gastroenterol. 1999;94(2):322–7. doi: 10.1111/j.1572-0241.1999.00849.x. doi: 10.1111/j.1572-0241.1999.00849.x. [DOI] [PubMed] [Google Scholar]
- 84.Correa-Gallego C, Fong Y, Gonen M, et al. A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Ann Surg Oncol. 2014;21(13):4278–83. doi: 10.1245/s10434-014-3817-0. doi: 10.1245/s10434-014-3817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leung U, Kuk D, D’Angelica MI, et al. Long-term outcomes following microwave ablation for liver malignancies. Br J Surg. 2015;102(1):85–91. doi: 10.1002/bjs.9649. doi: 10.1002/bjs.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Groeschl RT, Pilgrim CHC, Hanna EM, et al. Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg. 2014;259(6):1195–200. doi: 10.1097/SLA.0000000000000234. doi: 10.1097/SLA.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 87.Yu NC, Raman SS, Kim YJ, Lassman C, Chang X, Lu DSK. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol. 2008;19(7):1087–92. doi: 10.1016/j.jvir.2008.03.023. doi: 10.1016/j.jvir.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 88.Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236(1):132–9. doi: 10.1148/radiol.2361031249. doi: 10.1148/radiol.2361031249. [DOI] [PubMed] [Google Scholar]
- 89.Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25(1):S69–83. doi: 10.1148/rg.25si055501. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 90•.Stättner S, Primavesi F, Yip VS, et al. Evolution of surgical micro-wave ablation for the treatment of colorectal cancer liver metastasis: review of the literature and a single centre experience. Surg Today. 2014;45(4):407–15. doi: 10.1007/s00595-014-0879-3. doi: 10.1007/s00595-014-0879-3. This study shows the feasibility and safety of laparoscopic MWC.