Abstract
Background
Immunoglobulin A nephropathy (IgAN) is one of most common forms of glomerulonephritis. At this point, the clinical impact of hyperuricemia on IgAN is not clear. The aim of the present study was to explore the clinical impact of hyperuricemia on the progression of IgAN.
Study Design
Multicenter retrospective cohort study.
Setting & Participants
935 IgAN patients who were diagnosed by kidney biopsy at Osaka University Hospital, Osaka General Hospital, and Osaka Rosai Hospital. were included in this study.
Predictor
Uric acid levels at renal biopsy.
Outcomes
The outcome of interest was the time from the kidney biopsy to the time when a 50% increase in the baseline serum creatinine level was observed, which was defined as "progression".
Measurements
The baseline characteristics according to the kidney biopsy at the time of diagnosis were collected from the medical records, and included age, gender, body mass index, hypertension, diabetes (use of antidiabetic drugs), serum levels of creatinine, urinary protein, smoking status, RAAS blockers and steroid therapy.
Results
An elevated serum uric acid level was an independent risk factor for progression in female patients (per 1.0 mg/dL, multivariate-adjusted incident rate ratio 1.33 [95% confidence interval 1.07, 1.64], P = 0.008) but not in male patients (1.02 [0.81, 1.29], P = 0.855). To control a confounding effect of renal function on an association between serum uric acid level and progression in female patients, age- and serum creatinine-matched and propensity score-matched analyses were performed, and these results also supported the effect by uric acid on kidney disease progression independent of basal kidney function.
Limitations
A cohort analyzed retorospectively.
Conclusions
This study revealed that an elevated uric acid level was an independent risk factor for ESKD in female IgAN patients. Therefore, uric acid might be a treatable target in female IgAN patients.
Introduction
Immunoglobulin A nephropathy (IgAN) is one of most common forms of glomerulonephritis [1], and the 30-year renal survival rate has been reported to be 50.3% [2], which indicates that its prognosis is poor. Several types of factors are related to the pathogenesis of IgAN, such as genetic factors [3,4], immune factors [5–7], and infectious factors [8,9]. In addition to the classical renal progression factors, including hypertension, proteinuria, and decreased renal function, other factors that are related to the renal progression of IgAN have been reported, such as smoking [10,11], hypertriglyceridemia [12], hyperuricemia [12], and atherosclerosis-related genetic factors [4,10,13,14].
Serum uric acid level has been considered to be an atherosclerotic factor [15–18]. Moreover, many reports have suggested that females are at a higher risk for uric acid-induced atherosclerotic diseases than males, whereas no evidence indicates that males experience more effects from uric acid than females. Indeed, associations that have been found between the serum uric acid level and the incidence of coronary heart disease [19], hypertension [17], and kidney dysfunction [20,21] were stronger in women than in men. Uric acid is secreted in the kidney, and the deterioration of kidney function should lead to high uric acid levels. Therefore, if the association between the glomerular filtration rate (GFR) and uric acid was not included in a given analysis, the effect of uric acid on renal function might have been overestimated. To support the idea that uric acid may be a predictor of renal function in patients with IgAN, a Finnish IgAN cohort study reported that the IgAN group with progression of renal disease had a higher baseline serum uric acid level than those in the IgAN group with stable renal function. However, the baseline renal function was not included in many statistical analyses [12]. Moreover, the authors of this report did not determine the gender differences with respect to the uric acid level, even though females are expected to be at a higher risk from the effects of uric acid than males. At this point, the clinical impact of hyperuricemia on IgAN is not clear.
The aim of the present study was to explore the clinical impact of hyperuricemia on the progression of IgAN. This multicenter observational cohort study was organized by a research group for the Study of Outcomes and Practice patterns of primary Immunoglobulin A Nephropathy (STOP-IgAN), which is based out of three major nephrology centers in Osaka, Japan.
Methods
Patients
The candidate patients of the present study were included in our previous retrospective cohort study: the Study of Outcomes and Practice patterns of IgA Nephropathy (STOP-IgAN). The study protocol was described in detail elsewhere [13]. Briefly, between 1992 and 2005, 1001 patients aged at least 15 years were diagnosed with IgAN by kidney biopsy at Osaka University Hospital, Osaka General Hospital, and Osaka Rosai Hospital. The informed consent for the kidney biopsy, on behalf of patients between 15 to 20, was always done accompanied with their parent, and written informed consent for kidney biopsy was obtained from parents. This procedure obeyed the Japanese Low. After the exclusion of 29 (2.9%) patients who were treated with uric acid-lowering agents at the time of kidney biopsy and 37 (3.7%) patients with missing data, the present study finally included 935 (93.4%) patients. The patients were followed-up until June 2009. The study protocol was approved by the ethics committees (Osaka University Hospital Ethical Committee, Osaka Rousai Hosipital Ethical Committee, Osaka general medical center ethical committee) at the three hospitals (Osaka University Hospital, Osaka Rousai Hospital, Osaka general medical center). The procedure for informed consent was that our study protocol was open to public in our homepage (http://www.med.osaka-u.ac.jp/pub/kid/kid/studyPlan/stopigan.htm), and if the participants would not want to be included in this study, they can contact with us. There was no patient who wanted not to be included in this study. We used the clinical renal biopsy database which each hospital had for clinical use to build up the IgA nephropathy cohort in each hospital. The all data were de-identified with linking capacity within each facility before the data from three hospitals were combined and authors made statistical analysis. We cut the patient name, the day of birth (except month, year of birth), patient ID in each hospital, labeled the patients in each hospital, and made the correspondence table and the table was kept in the locked area. Data are available from the Osaka University Institutional Data Access / Ethics Committee for researchers who meet the criteria for access to confidential data (rinri@hp-crc.med.osaka-u.ac.jp, Osaka University Hospital, Future medical Center, Division of clinical Trial, Yamadaoka2-2, Suita, Osaka, Japan)
Measurements
The baseline characteristics according to the kidney biopsy at the time of diagnosis were collected from the medical records, and included age, gender, body mass index, hypertension (systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, and/or use of antihypertensive drugs), diabetes (use of antidiabetic drugs), serum levels of creatinine and uric acid, urinary protein, smoking status (current, past, or non-smokers), and the number of cigarettes smoked daily at the time of the kidney biopsy (0 for past smokers and non-smokers). At two hospitals, the level of serum creatinine was measured by the enzymatic method during the entire observational period, whereas the measurement of creatinine levels was changed from the Jaffe method to the enzymatic method in November 1995 at one hospital. Using sera from 648 patients who were randomly selected from the hospital, the correlation coefficient r between creatinine values that was measured by the Jaffe method and the enzymatic method was 0.998; the least squares method determined the following predictive equation: creatinine (enzymatic method) = 0.94 X creatinine (Jaffe method)—0.25. Thus, the creatinine values for the 205 patients who were diagnosed with IgAN by kidney biopsy before November 1995 at the three hospitals were calibrated using this equation. The antihypertensive drugs that were used by the patients were as follows: RAAS blockers (angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aldosterone receptor blockers), calcium channel blockers, alpha-blockers, beta-blockers, and thiazides. Information on the smoking status and the number of cigarettes smoked daily was obtained via a questionnaire when the patients were admitted to the hospital for the kidney biopsy.
Information on the use of RAAS blockers and corticosteroids initiated within one year of the kidney biopsy was also collected to determine any therapeutic interventions.
Outcome
The outcome of interest was the time from the kidney biopsy to the time when a 50% increase in the baseline serum creatinine level was observed, which was defined as "progression". Serum creatinine was measured as required according to the clinical needs of each patient. The patients were followed-up until June 2009 and were censored on the last day of measurement of serum creatinine before June 2009.
Statistics
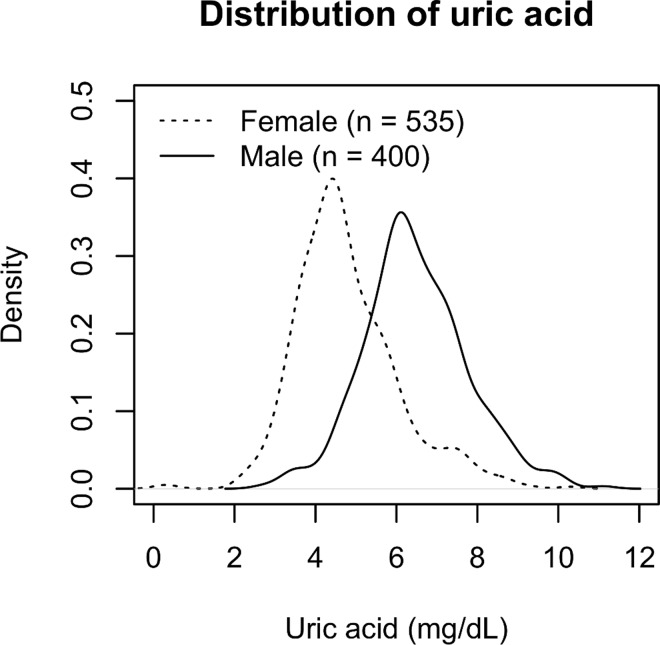
The distributions of the serum uric acid levels in the male and female patients were described with the kernel density estimation and were compared using unpaired t-test. Because the male and female distributions of uric acid were substantially different (Fig 1), all subsequent analyses were conducted separately in males and females. After the categorization of the baseline uric acid level into male and female quartiles, the differences in their clinical characteristics were compared using ANOVA, the Kruskal-Wallis test or the chi-square test, as appropriate. The cumulative probability of progression was calculated with the Kaplan-Meier method. An association between the serum uric acid level and progression was assessed by log-rank test and the Poisson regression model, which were adjusted for clinically relevant factors. The appropriateness of the Poisson regression model was tested with a goodness-of-fit test using deviance statistics.
Fig 1. Kernel density estimates of serum uric acid levels after kidney biopsy of 535 female and 400 male patients.
To control the confounding effect of renal function on an association between serum uric acid level and progression, each patient in the first (Q1) and second (Q2) quartiles of serum uric acid level at kidney biopsy were randomly matched with a patient in its third (Q3) and fourth (Q4) quartiles at a ratio of 1:1 without replacement, using two distinct matching methods. In the first method, patients were matched by baseline age (±5 years) and serum creatinine (±0.1 mg/dL). The second method used a propensity score, an estimated probability of being a Q3 –Q4 patient, given the observed confounding variables [22]. The propensity score for each patient was calculated in a separate multivariate logistic regression model including the baseline confounding factors (age, body mass index, hypertension, serum creatinine level, urinary protein excretion, the number of cigarettes smoked daily, and use of antidiabetic drugs) and use of RAAS blockers and corticosteroids initiated within one year of the kidney biopsy as independent variables. Calibration was assessed using Hosmer-Lemeshow goodness-of-fit test. Each Q1 –Q2 patient were matched to Q3 –Q4 patient with the closest propensity score, using a greedy matching algorithm with a caliper width of 0.2 standard deviation of the logit the propensity score [23]. In the age- and serum creatinine-matched pairs and the propensity score-matched pairs, baseline characteristics at kidney biopsy and therapeutic interventions within 1 year of kidney biopsy were compared, using paired t-test, Wilcoxon singed rank test, or McNemar test, as appropriate. Incident ratio ratio (IRR) of Q3 –Q4 of serum uric acid level (vs. Q1 –Q2) was calculated using Poisson regression model.
Statistical significance was set at P < 0.05. Statistical analyses were performed using R version 3.2.4 "Very Secure Dishes" (The R Foundation for Statistical Computing, www.r-project.org).
Results
The mean baseline serum uric acid levels of the 535 female and 400 male patients at the time of diagnosis by kidney biopsy were 4.8±1.3 (mean±SD) and 6.5±1.3 mg/dL, respectively (P < 0.001) (Fig 1). Their clinical characteristics that were stratified into quartiles of the baseline serum uric acid level are described in Tables 1 and 2, respectively. In female patients, a significant difference was observed in the following parameters among quartiles of the baseline serum uric acid level: age, body mass index, hypertension, serum creatinine, urinary protein, the number of cigarettes smoked daily by current smokers at the time of diagnosis by kidney biopsy, and therapeutic interventions (RAAS blockade and the use of corticosteroids) within one year of kidney biopsy (Table 1). In male patients, similar differences were observed, except in the baseline number of cigarettes smoked daily by current smokers and therapeutic interventions (RAAS blockade within 1 year of kidney biopsy) (Table 2).
Table 1. Clinical characteristics of 535 female patients with IgAN stratified in quartiles of uric acid levels.
| Q1 | Q2 | Q3 | Q4 | P | |
|---|---|---|---|---|---|
| Uric acid (mg/dL) | 3.6 (0.2–3.9) | 4.3 (4.0–4.6) | 5.0 (4.7–5.4) | 6.2 (5.5–10.3) | |
| Number | 140 | 146 | 118 | 131 | |
| Baseline characteristics at renal biopsy | |||||
| Age (years) | 27 (22, 37) | 28 (23, 44) | 29 (22, 45) | 44 (30, 53) | <0.001 |
| Body mass index (kg/m2) | 20.6±2.7 | 21.0±3.0 | 21.9±3.9 | 24.0±3.9 | <0.001 |
| Hypertension (%)§ | 10.0 | 18.5 | 21.2 | 49.6 | <0.001 |
| Serum creatinine (mg/dL) | 0.60 (0.51, 0.69) | 0.60 (0.59, 0.70) | 0.68 (0.60, 0.80) | 0.85 (0.70, 1.07) | <0.001 |
| Urinary protein (g/day) | 0.24 (0.13, 0.53) | 0.30 (0.16, 0.62) | 0.44 (0.18, 0.86) | 0.64 (0.32, 1.70) | <0.001 |
| Current smoker (%) | 12.1 | 14.4 | 12.7 | 16.0 | 0.448 |
| Cigarettes (per day)# | 6 (5, 10) | 15 (10, 20) | 15 (10, 20) | 20 (10, 20) | 0.297 |
| Diabetes (%) | 0.8 | 0.7 | 0.8 | 0.8 | 0.927 |
| Therapeutic interventions within 1 year of kidney biopsy | |||||
| RAAS blockade (%) | 25.0 | 21.9 | 40.7 | 60.3 | <0.001 |
| Use of corticosteroids (%) | 18.6 | 28.8 | 41.5 | 26.0 | 0.047 |
Median (min—max), median (25%, 75%), mean±SD.
RAAS, renin-angiotensin aldosterone system
§Systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, and/or use of antihypertensive drugs at the time of kidney biopsy
#Number of cigarettes smoked in current smokers at the time of kidney biopsy was displayed. P value was calculated after including past and non-smokers with 0 cigarette per day.
Table 2. Clinical characteristics of 400 male patients with IgAN stratified in quartiles of uric acid levels.
| Q1 | Q2 | Q3 | Q4 | P | |
|---|---|---|---|---|---|
| Uric acid (mg/dL) | 5.2 (2.7–5.7) | 6.1 (5.8–6.4) | 6.8 (6.5–7.2) | 8.0 (7.3–11.1) | |
| Number | 104 | 108 | 88 | 100 | |
| Baseline characteristics at renal biopsy | |||||
| Age (years) | 28 (20, 47) | 28 (21, 46) | 30 (22, 45) | 37 (26, 52) | 0.002 |
| Body mass index (kg/m2) | 21.6±2.9 | 22.8±3.2 | 23.0±3.2 | 24.3±3.6 | <0.001 |
| Hypertension (%)§ | 28.8 | 32.4 | 36.4 | 47.0 | 0.006 |
| Serum creatinine (mg/dL) | 0.80 (0.73, 0.90) | 0.87 (0.73, 1.00) | 0.90 (0.78, 1.01) | 1.07 (0.90, 1.20) | <0.001 |
| Urinary protein (g/day) | 0.31 (0.15, 0.68) | 0.37 (0.19, 0.73) | 0.41 (0.22, 0.96) | 0.62 (0.36, 1.35) | <0.001 |
| Current smoker (%) | 31.7 | 29.6 | 29.5 | 36.0 | 0.530 |
| Cigarettes (per day)# | 20 (10, 25) | 20 (15, 26) | 20 (10, 20) | 20 (20, 33) | 0.350 |
| Diabetes (%) | 1.9 | 0.9 | 1.1 | 4.0 | 0.292 |
| Therapeutic interventions within 1 year of kidney biopsy | |||||
| RAS blockade (%) | 44.2 | 51.9 | 44.3 | 59.0 | 0.092 |
| Use of corticosteroids (%) | 18.3 | 32.4 | 21.6 | 34.0 | 0.060 |
Median (min—max), median (25%, 75%), mean±SD.
RAS, renin-angiotensin system
§Systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, and/or use of antihypertensive drugs at kidney biopsy
#Number of cigarettes smoked in current smokers at the time of kidney biopsy was displayed. P value was calculated after including past and non-smokers with 0 cigarette per day.
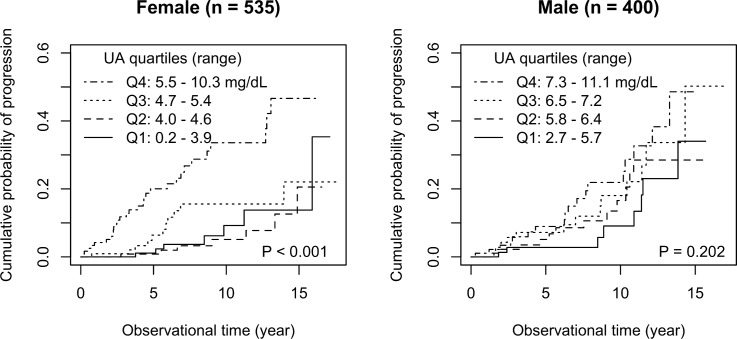
In female patients, during the median 5.7 years (interquartile range 2.6, 9.3) of the observational period, 7 (5.0%), 7 (4.8%), 11 (9.3%), and 30 (22.9%) patients in Q1, Q2, Q3, and Q4 of serum uric acid levels developed progression, respectively (P < 0.001). Female patients in the higher quartiles of serum uric acid levels were at a higher risk of progression (the cumulative probabilities of progression at 5 and 10 years were 0.01 [95% confidence interval 0.00, 0.03] and 0.09 [0.00, 0.17] in Q1 patients, 0.01 [0.00, 0.02] and 0.05 [0.00, 0.10] in Q2 patients, 0.06 [0.01, 0.12] and 0.16 [0.06, 0.24] in Q3 patients, and 0.20 [0.12, 0.28] and 0.34 [0.21, 0.44] in Q4 patients, respectively (P < 0.001)) (Fig 2). On the contrary, in male Q1, Q2, Q3, and Q4 patients, progression was observed in 8 (7.7%), 12 (11.1%), 13 (14.8%), and 18 (18.0%) patients, respectively, during the 5.2 years (interquartile range 2.1, 9.8) of the observational period (P = 0.141). No significant difference was observed in the cumulative probabilities of progression among Q1, Q2, Q3, and Q4 patients (cumulative probabilities of progression at 5 and 10 years was 0.03 [95% confidence interval 0.00, 0.06] and 0.09 [0.00, 0.18] in Q1 patients, 0.05 [0.00, 0.10] and 0.17 [0.05, 0.27] in Q2 patients, 0.07 [0.01, 0.13] and 0.18 [0.06, 0.29] in Q3 patients, and 0.09 [0.02, 0.15] and 0.22 [0.10, 0.32] in Q4 patients, respectively (P = 0.202)) (Fig 2).
Fig 2. Cumulative probabilities of a 150% increase in the serum creatinine level (progression) in 535 female and 400 male patients who were stratified into quartiles based on uric acid level.
In a univariate Poisson regression model, the serum uric acid level was significantly associated with progression in female patients (per 1.0 mg/dl, IRR 1.82 [95% confidence interval 1.54, 2.13], P < 0.001) (Table 3). Even after adjustment for clinically relevant factors, an association between the serum uric acid level and progression was still significant, although its association was attenuated (1.33 [1.07, 1.64], P = 0.008 in multivariate model 1; 1.34 [1.05, 1.68], P = 0.015 in model 2 in Table 3). An elevated serum uric acid level was identified to be a significant predictor of progression in female patients, together with older age, and elevated levels of serum creatinine and proteinuria. In regard to male patients, the serum uric acid level was associated with progression at a marginally significant level according to a univariate model (1.20 [0.97, 1.47], P = 0.089). However, a multivariate adjustment for clinically relevant factors blunted this association (1.02 [0.81, 1.29], P = 0.855 in model 1; 1.04 [0.80, 1.34], P = 0.787 in model 2 in Table 3). Elevated serum levels of serum creatinine and proteins in the urine, the number of cigarettes smoked daily, and the presence of diabetes were identified to be significant predictors of progression in male patients.
Table 3. Predictors of progression in 535 female and 400 male patients with IgAN.
| Univariate model | Multivariate model 1 | Multivariate model 2 | ||||
|---|---|---|---|---|---|---|
| IRR (95%CI) | P | IRR (95%CI) | P | IRR (95%CI) | P | |
| Predictors in female patients (n = 533) | ||||||
| Age (per 10 years) | 1.53 (1.27, 1.85) | <0.001 | 1.27 (1.02, 1.57) | 0.032 | 1.29 (1.02, 1.62) | 0.035 |
| Body mass index (per 1.0 kg/m2) | 1.05 (0.98, 1.12) | 0.121 | 0.97 (0.88, 1.05) | 0.445 | ||
| Hypertension | 3.24 (1.91, 5.52) | <0.001 | 1.32 (0.71, 2.48) | 0.380 | 1.30 (0.68, 2.46) | 0.423 |
| Serum creatinine (per 1.0 mg/dL) | 4.07 (3.15, 4.98) | <0.001 | 2.60 (1.80, 3.48) | <0.001 | 2.72 (1.85, 3.73) | <0.001 |
| Urinary protein (per 1.0 g/day) | 1.44 (1.27, 1.60) | <0.001 | 1.20 (1.03, 1.37) | 0.008 | 1.26 (1.07, 1.46) | 0.003 |
| Uric acid (per 1.0 mg/dL) | 1.82 (1.54, 2.13) | <0.001 | 1.33 (1.07, 1.64) | 0.008 | 1.34 (1.05, 1.68) | 0.015 |
| Cigarettes (per 20/day)# | 1.85 (0.76, 3.83) | 0.130 | 1.39 (0.63, 2.63) | 0.361 | ||
| Diabetes | 2.13 (0.12, 9.67) | 0.454 | 1.25 (0.07, 6.21) | 0.832 | ||
| RAS blockade | 1.96 (1.15, 3.34) | 0.013 | 0.86 (0.46, 1.60) | 0.632 | ||
| Use of corticosteroids | 0.92 (0.48, 1.65) | 0.792 | 0.61 (0.29, 1.21) | 0.178 | ||
| Predictors in male patients (n = 400) | ||||||
| Age (per 10 years) | 1.21 (1.00, 1.47) | 0.048 | 1.09 (0.88, 1.34) | 0.427 | 0.91 (0.73. 1.15) | 0.424 |
| Body mass index (per 1.0 kg/m2) | 1.01 (0.93, 1.09) | 0.812 | 0.96 (0.87, 1.07) | 0.482 | ||
| Hypertension | 1.52 (0.88, 2.65) | 0.133 | 1.15 (0.64, 2.07) | 0.637 | 1.17 (0.62, 2.21) | 0.623 |
| Serum creatinine (per 1.0 mg/dL) | 3.24 (1.75, 5.07) | <0.001 | 2.36 (1.14, 4.17) | 0.008 | 2.89 (1.34, 5.65) | 0.003 |
| Urinary protein (per 1.0 g/day) | 1.44 (1.25, 1.63) | <0.001 | 1.39 (1.20, 1.59) | <0.001 | 1.42 (1.20, 1.66) | <0.001 |
| Uric acid (per 1.0 mg/dL) | 1.20 (0.97. 1.47) | 0.089 | 1.02 (0.81, 1.29) | 0.855 | 1.04 (0.80, 1.34) | 0.787 |
| Cigarettes (per 20/day)# | 1.90 (1.39, 2.53) | <0.001 | 1.88 (1.31, 2.63) | <0.001 | ||
| Diabetes | 7.28 (2.20, 17.88) | <0.001 | 8.88 (2.48, 24.91) | <0.001 | ||
| RAS blockade | 1.22 (0.70, 2.17) | 0.496 | 0.90 (0.48, 1.69) | 0.737 | ||
| Use of corticosteroids | 0.79 (0.41, 1.45) | 0.466 | 0.56 (0.26, 1.11) | 0.108 | ||
CI, confidence interval; IRR, incident rate ratio.
To clarify an association between the serum uric acid level and progression, IRR of each quartile of serum uric acid levels was calculated. In females, compared with Q2, Q4 was significantly associated with progression in a univariate model (Q1, 1.26 [0.43, 3.69], P = 0.662; Q2, 1.00 [reference]; Q3, 2.25 [0.89, 6.11], P = 0.094; Q4, 6.16 [2.87, 15.27], P < 0.001) (univariate model in Table 4). Even after an adjustment for age, hypertension, serum creatinine, and urinary protein, Q4 was significantly associated with progression, but these covariates greatly attenuated the magnitude of the association between the level of serum uric acid and progression (Q1, 1.40 [0.48, 4.09], P = 0.533; Q2, 1.00 [reference]; Q3, 1.82 [0.71, 4.98], P = 0.217; Q4, 2.56 [1.10, 6.70], P = 0.039) (multivariate model 1 in Table 4). Further adjustment for body mass index, the number of cigarettes, the presence of diabetes at the time of kidney biopsy, and therapeutic interventions showed a similar result (multivariate model 2 in Table 4). In male patients, no significant association was observed between the quartiles of serum uric acid levels and progression (Table 4).
Table 4. Associations between uric acid and progression.
| Univariate model | Multivariate model 1† | Multivariate model 2‡ | ||||
|---|---|---|---|---|---|---|
| IRR (95%CI) | P | IRR (95%CI) | P | IRR (95%CI) | P | |
| Quartiles of uric acid levels in female patients (n = 533) | ||||||
| Q1: 3.6 (0.2–3.9) mg/dL | 1.26 (0.43, 3.69) | 0.662 | 1.40 (0.48, 4.09) | 0.533 | 1.30 (0.44, 3.86) | 0.624 |
| Q2: 4.3 (4.0–4.6) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Q3: 5.0 (4.7–5.2) | 2.25 (0.89, 6.11) | 0.094 | 1.82 (0.71, 4.98) | 0.217 | 1.99 (0.77, 5.52) | 0.163 |
| Q4: 6.2 (5.8–7.2) | 6.16 (2.87, 15.27) | <0.001 | 2.56 (1.10, 6.70) | 0.039 | 2.58 (1.05, 7.05) | 0.049 |
| Quartiles of uric acid levels in male patients (n = 400) | ||||||
| Q1: 5.2 (2.7–5.7) mg/dL | 0.78 (0.30, 1.88) | 0.580 | 0.92 (0.36, 2.25) | 0.854 | 0.85 (0.32, 2.14) | 0.727 |
| Q2: 6.1 (5.8–6.2) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Q3: 6.8 (6.5–7.2) | 1.33 (0.60, 2.96) | 0.475 | 1.26 (0.57, 2.79) | 0.570 | 1.45 (0.65, 3.30) | 0.363 |
| Q4: 8.0 (7.3–11.1) | 1.64 (0.80, 3.49) | 0.185 | 0.99 (0.46, 2.20) | 0.984 | 1.10 (0.48, 2.53) | 0.826 |
Median (min—max)
CI, confidence interval; IRR, incident rate ratio
†Adjusted for age, hypertension, serum creatinine, and urinary protein at the time of kidney biopsy.
‡Adjusted for the covariates in model 1 plus body mass index, the number of cigarettes, diabetes at kidney biopsy, renin-angiotensin system blockade and the use of corticosteroids within one year of kidney biopsy.
To control a confounding effect of renal function on an association between serum uric acid level and progression in female patients, 173 patients in Q1 and Q2 were randomly matched to 173 patients in Q3 and Q4 by age (±5 years) and serum creatinine level (±0.1 mg/dL) (Table 5). Significant differences were observed in baseline body mass index, serum creatinine level, and urinary protein, and use of corticosteroid within 1 year of kidney biopsy, although serum creatinine level was, clinically, not at significant level (Q1 –Q2, median 0.60 mg/dL [interquartile range 0.58, 0.72] vs. Q3 –Q4, 0.68 [0.60, 0.78], P <0.001). Compared with Q1 –Q2, Q3 –Q4 was significantly associated with progression in univariate model (IRR 2.87 [1.18, 8.00], P<0.001), multivariate model 1 (2.69 [1.06, 7.70], P = 0.046), and multivariate model 2 (2.97 [1.07–9.31], P = 0.046) similarly (Table 6).
Table 5. Clinical characteristics of age- and serum creatinine-matched female patients (n = 346).
| Q1—Q2 | Q3—Q4 | P | |
|---|---|---|---|
| Uric acid (mg/dL) | 4.0 (0.2–4.6) | 5.3 (4.7–8.6) | |
| Number | 173 | 173 | |
| Baseline characteristics at renal biopsy | |||
| Age (years) | 29 (23, 43) | 29 (22, 44) | 0.713 |
| Body mass index (kg/m2) | 20.7±3.0 | 22.7±4.3 | <0.001 |
| Hypertension (%)§ | 18.5 | 21.4 | 0.551 |
| Serum creatinine (mg/dL) | 0.60 (0.58, 0.72) | 0.68 (0.60, 0.78) | <0.001 |
| Urinary protein (g/day) | 0.30 (0.13, 0.60) | 0.46 (0.20, 0.95) | <0.001 |
| Current smoker (%) | 12.7 | 13.9 | 0.871 |
| Cigarettes (per day)# | 10 (5, 20) | 15 (10, 20) | 0.406 |
| Diabetes (%) | 1.0 | 0.0 | |
| Therapeutic interventions within 1 year of kidney biopsy | |||
| RAAS blockade (%) | 23.1 | 45.7 | <0.001 |
| Use of corticosteroids (%) | 25.4 | 34.1 | 0.919 |
Median (min—max), median (25%, 75%), mean±SD.
RAAS, renin-angiotensin aldosterone system
§Systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, and/or use of antihypertensive drugs at the time of kidney biopsy
#Number of cigarettes smoked in current smokers at the time of kidney biopsy was displayed. P value was calculated after including past and non-smokers with 0 cigarette per day.
Table 6. Associations between uric acid and progression in matched female patients.
| Quartiles of baseline uric acid | ||||
|---|---|---|---|---|
| Q1—Q2 | Q3—Q4 | |||
| Age- and serum creatinine-matched patients (n = 346) | ||||
| Baseline uric acid (mg/dL) | 4.0 (0.2–4.6) | 5.3 (4.7–8.6) | ||
| Univariate model | IRR (95% CI) | 1.00 (reference) | 2.87 (1.18, 8.00) | <0.001 |
| Multivariate model 1† | 1.00 (reference) | 2.69 (1.06, 7.70) | 0.046 | |
| Multivariate model 2‡ | 1.00 (reference) | 2.97 (1.07, 9.31) | 0.046 | |
| Propensity score-matched patients (n = 284) | ||||
| Baseline uric acid (mg/dL) | 4.1 (2.2–4.6) | 5.3 (4.7–9.3) | ||
| Univariate model | IRR (95% CI) | 1.00 (reference) | 3.09 (1.18, 9.57) | 0.030 |
Median (min—max)
CI, confidence interval; IRR, incident rate ratio
†Adjusted for age, hypertension, serum creatinine, and urinary protein at the time of kidney biopsy.
‡Adjusted for the covariates in model 1 plus body mass index, the number of cigarettes, diabetes at kidney biopsy, renin-angiotensin system blockade and the use of corticosteroids within one year of kidney biopsy.
Between propensity score-matched pairs (n = 284), no significant difference was observed in baseline characteristics and therapeutic interventions within 1 year of kidney biopsy (Table 7). Even after propensity score-matching, Q3 –Q4 patients were at significantly higher risk of progression, compared with Q1 –Q2 patients (IRR 3.09 [1.18, 9.57], P = 0.030) (Table 6).
Table 7. Clinical characteristics of propensity score-matched patients (n = 284).
| Q1–2 | Q3–4 | P | |
|---|---|---|---|
| Uric acid (mg/dL) | 4.1 (2.2–4.6) | 5.3 (4.7–9.3) | |
| Number | 142 | 142 | |
| Baseline characteristics at renal biopsy | |||
| Age (years) | 31 (24, 44) | 31 (23, 46) | 0.515 |
| Body mass index (kg/m2) | 21.6±3.2 | 21.8±3.3 | 0.514 |
| Hypertension (%)§ | 23.9 | 21.1 | 0.671 |
| Serum creatinine (mg/dL) | 0.60 (0.59, 0.75) | 0.67 (0.60, 0.78) | 0.231 |
| Urinary protein (g/day) | 0.37 (0.16, 0.70) | 0.39 (0.19, 0.70) | 0.580 |
| Current smoker (%) | 17.6 | 12.0 | 0.256 |
| Cigarettes (per day)# | 10 (5, 20) | 15 (10, 20) | 0.449 |
| Diabetes (%) | 0.7 | 0.0 | |
| Therapeutic interventions within 1 year of kidney biopsy | |||
| RAAS blockade (%) | 35.9 | 36.6 | 1.000 |
| Use of corticosteroids (%) | 27.5 | 29.6 | 0.787 |
Median (min—max), median (25%, 75%), mean±SD.
RAAS, renin-angiotensin aldosterone system
§Systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, and/or use of antihypertensive drugs at the time of kidney biopsy
#Number of cigarettes that were smoked daily by current smokers at the time of kidney biopsy was displayed. P value was calculated after including past and non-smokers with 0 cigarette per day.
Discussion
This retrospective observational study of 935 patients with IgAN revealed that an elevated serum uric acid level was an independent risk factor for progression in female patients (per 1.0 mg/dL, multivariate-adjusted IRR 1.33 [95% confidence interval 1.07, 1.64], P = 0.008) but not in male patients (1.02 [0.81, 1.29], P = 0.855). To control a confounding effect of renal function on an association between serum uric acid level and progression in female patients, age- and serum creatinine-matched and propensity score-matched analyses were performed, and these results also supported the effect by uric acid on kidney disease progression independent of basal kidney function. Two of the advantages of the present study were a very large cohort and a long observational period. Those advantages enabled us to assess an association between the serum uric acid level and the progression of IgAN in male and female patients separately, after adjustment for clinically relevant factors including the serum creatinine level, which is an index of renal function.
A limited number of cohort studies have reported an association between the serum uric acid level and progression of IgAN. A Finnish cohort study first showed that hyperuricemia (defined as > 7.56 mg/dl in males and > 5.55 mg/dl in females) was a risk factor for the progression of IgAN, but this result had not been adjusted for kidney function [12]. Ohno et al reported that uric acid impacted renal prognosis in IgA nephropathy but did not provide an analysis of the gender differences [24]. Shi-Y et al also confirmed that uric acid was a risk factor for progression in 353 patients with IgA nephropathy in a retrospective cohort, but again, an analysis of the gender differences was not included [25]. Recently, uric acid was determined to be a risk factor for renal dysfunction only in patients with IgAN with stage G3a CKD [26]. Our study revealed the effect of uric acid upon disease progression in female patients with IgAN.
Our results indicated a gender difference with respect to the effects of uric acid on the progression of uric acid-induced kidney disease. The mechanism of this gender difference in the association between the serum uric acid level and the progression of IgAN remains unclear. Estrogen suppressed the protein levels of the urate reabsorptive transporter (urate transporter 1; URAT1) in the kidney, which resulted in an increase in uric acid excretion and a decrease in the uric acid level in the serum [27]. Indeed, postmenopausal women have higher serum uric acid levels, and the use of postmenopausal hormones (e.g., estrogen analogs) is associated with lower levels of uric acid [28]. After natural menopause, women experience an increase in uric acid levels and an increase in the risk of atherosclerotic diseases [29,30]. Female IgAN patients with high uric acid levels might have a similar atherosclerotic condition as menopausal women with high levels of uric acid and low levels of estrogen. Moreover, renal arteriolar hyalinosis could be significantly highly observed in female subjects with a uric acid level of more than 5 mg/dl, whereas the hyalinosis lesions were observed in male subjects with a uric acid level of more than 7 mg/dl [31]. These findings suggest that females might be more vulnerable to uric acid-induced organ damage, which might coincide with the phenomenon that uric acid levels in females were within a lower range than those in males. In terms of anti-arteriosclerosis therapy, use of satin for IgA nephropathy female patients with hyper uric acid might be useful. Many report including meta-analysis supported the renal protective effect of statin [32,33]. While information about the statin use was not available, average age of IgA nephropathy cohort in this study was 31years old, and average total cholesterol was 194mg/dl (5.03mmol/L), therefore, major part of this cohort might be not treated with statin. And also there were very few atherosclerotic events to detect the gender differences of CVD events due to relative young age of this cohort, although some reports support gender difference of CVD events associated hyper uric acids [17,19–21].
Several interventional studies have demonstrated that the treatment of hyperuricaemia ameliorated the progression of CKD [18]. A Chinese randomized controlled trial that included 54 hyperuricemic patients with CKD, demonstrated that allopurinol decreased systolic blood pressure and slowed the progression of CKD [18]. Through a non-randomized interventional study, Kanbay et al also reported this favorable effect of uric acid-lowering therapy on blood pressure and GFR in patients with stage G2 CKD [34]. Additionally, the treatment of 40 IgA nephropathy patients with allopurinol decreased their blood pressure, although the small number of patients and the short observational period hindered a statistically meaningful assessment of any renoprotective effect of allopurinol [25]. This reduction in the progression of CKD by uric acid-lowering therapy was considered to be induced by an improvement in endothelial function [15,18]. Endothelial cells are one of the important cell types in the glomeruli, and they are considered to be related to hypertension and renal progression [35]. Our results suggested that hyperuricemia might be a treatable target in patients with IgAN, especially in female patients.
Our study has several limitations. First, this study did not include information in regard to uric acid-lowering therapy during the observational periods, although patients with IgAN who received this therapy at the time of renal biopsy were excluded from this study. The majority of patients in this study had uric acid levels that were lower than those in the treatable range; therefore, this effect might be small. Second, although all patients were diagnosed with IgAN by kidney biopsy, histological findings were not included as covariates of interest in the present study, partly because each facility used different histological grading systems; each facility also changed their grading system during the 14 years of the long entry period between 1992 and 2005. Recently, plasma uric acid reported to have association with interstitial fibrosis and tubular atrophy in IgA nephropathy patients [36], while this report did not demonstrate histological gender difference. The possibility that effect upon kidney dysfunction caused by uric acid may be mediated by kidney histological change should be confirmed by further study. Third, this study did not include the alcohol drinking status of the patients. The PREVEND study reported that alcohol consumption is inversely associated with the risk of development of CKD [37]. In Japan, alcohol consumption in males was higher than that in females, and therefore, the renoprotective effect of alcohol might lead to improvement in renal prognosis and may elevate the levels of uric acid in male patients with IgA nephropathy.
In conclusion, this study revealed that an elevated uric acid level was an independent risk factor for ESKD in female IgAN patients. Therefore, uric acid might be a treatable target in female IgAN patients.
Acknowledgments
We thank the patients enrolled in this study. We thank Mrs. Chiaki Okamoto for assistance with data collection. This work was partially supported by Grant-in-Aid for Scientific Research(C) to YN (26461247) from Japan Society for promotion science. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
Data are available from the Osaka University Institutional Ethics Committee (rinri@hp-crc.med.osaka-u.ac.jp, Osaka University Hospital, Future medical Center, Division of clinical Trial, Yamadaoka2-2, Suita, Osaka, Japan).
Funding Statement
This work was supported by Grant-in-Aid for Scientific Research(C) to YN (26461247) from Japan Society for promotion science. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wyatt RJ, Julian BA (2013) IgA nephropathy. N Engl J Med 368: 2402–2414. 10.1056/NEJMra1206793 [DOI] [PubMed] [Google Scholar]
- 2.Moriyama T, Tanaka K, Iwasaki C, Oshima Y, Ochi A, Kataoka H, et al. (2014) Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One 9: e91756 10.1371/journal.pone.0091756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto R, Nagasawa Y, Shoji T, Katakami N, Ohtoshi K, Hayaishi-Okano R, et al. (2012) A candidate gene approach to genetic contributors to the development of IgA nephropathy. Nephrol Dial Transplant 27: 1020–1030. 10.1093/ndt/gfr369 [DOI] [PubMed] [Google Scholar]
- 4.Kiryluk K, Novak J, Gharavi AG (2013) Pathogenesis of immunoglobulin A nephropathy: recent insight from genetic studies. Annu Rev Med 64: 339–356. 10.1146/annurev-med-041811-142014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coppo R, Amore A, Peruzzi L, Vergano L, Camilla R (2010) Innate immunity and IgA nephropathy. J Nephrol 23: 626–632. [PubMed] [Google Scholar]
- 6.Iwatani H, Nagasawa Y, Yamamoto R, Iio K, Mizui M, Horii A, et al. (2014) CD16CD56 cells are a potential culprit for hematuria in IgA nephropathy. Clin Exp Nephrol. [DOI] [PubMed] [Google Scholar]
- 7.Iio K, Nagasawa Y, Iwatani H, Yamamoto R, Horii A, Okuzaki D, et al. (2010) Microarray analysis of tonsils in immunoglobulin A nephropathy patients. Biochem Biophys Res Commun 393: 565–570. 10.1016/j.bbrc.2010.01.120 [DOI] [PubMed] [Google Scholar]
- 8.Suzuki S, Nakatomi Y, Sato H, Tsukada H, Arakawa M (1994) Haemophilus parainfluenzae antigen and antibody in renal biopsy samples and serum of patients with IgA nephropathy. Lancet 343: 12–16. [DOI] [PubMed] [Google Scholar]
- 9.Nagasawa Y, Iio K, Fukuda S, Date Y, Iwatani H, Yamamoto R, et al. (2014) Periodontal disease bacteria specific to tonsil in IgA nephropathy patients predicts the remission by the treatment. PLoS One 9: e81636 10.1371/journal.pone.0081636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagasawa Y, Yamamoto R, Rakugi H, Isaka Y (2012) Cigarette smoking and chronic kidney diseases. Hypertens Res 35: 261–265. 10.1038/hr.2011.205 [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto R, Nagasawa Y, Shoji T, Iwatani H, Hamano T, Kawada N, et al. (2010) Cigarette smoking and progression of IgA nephropathy. Am J Kidney Dis 56: 313–324. 10.1053/j.ajkd.2010.02.351 [DOI] [PubMed] [Google Scholar]
- 12.Syrjanen J, Mustonen J, Pasternack A (2000) Hypertriglyceridaemia and hyperuricaemia are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant 15: 34–42. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto R, Nagasawa Y, Shoji T, Inoue K, Uehata T, Kaneko T, et al. (2009) A candidate gene approach to genetic prognostic factors of IgA nephropathy—a result of Polymorphism REsearch to DIstinguish genetic factors Contributing To progression of IgA Nephropathy (PREDICT-IgAN). Nephrol Dial Transplant. [DOI] [PubMed] [Google Scholar]
- 14.Kiryluk K, Li Y, Sanna-Cherchi S, Rohanizadegan M, Suzuki H, Eitner F, et al. (2012) Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet 8: e1002765 10.1371/journal.pgen.1002765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gustafsson D, Unwin R (2013) The pathophysiology of hyperuricaemia and its possible relationship to cardiovascular disease, morbidity and mortality. BMC Nephrol 14: 164 10.1186/1471-2369-14-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M, Hou W, Zhang X, Hu L, Tang Z (2014) Hyperuricemia and risk of stroke: a systematic review and meta-analysis of prospective studies. Atherosclerosis 232: 265–270. 10.1016/j.atherosclerosis.2013.11.051 [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Qin T, Chen J, Li Y, Wang L, Huang H, et al. (2014) Hyperuricemia and risk of incident hypertension: a systematic review and meta-analysis of observational studies. PLoS One 9: e114259 10.1371/journal.pone.0114259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feig DI (2014) Serum uric acid and the risk of hypertension and chronic kidney disease. Curr Opin Rheumatol 26: 176–185. 10.1097/BOR.0000000000000033 [DOI] [PubMed] [Google Scholar]
- 19.Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA (2010) Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 62: 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akasaka H, Yoshida H, Takizawa H, Hanawa N, Tobisawa T, Tanaka M, et al. (2014) The impact of elevation of serum uric acid level on the natural history of glomerular filtration rate (GFR) and its sex difference. Nephrol Dial Transplant 29: 1932–1939. 10.1093/ndt/gfu197 [DOI] [PubMed] [Google Scholar]
- 21.Iseki K, Iseki C, Kinjo K (2013) Changes in serum uric acid have a reciprocal effect on eGFR change: a 10-year follow-up study of community-based screening in Okinawa, Japan. Hypertens Res 36: 650–654. 10.1038/hr.2013.11 [DOI] [PubMed] [Google Scholar]
- 22.Connors AF Jr., Speroff T, Dawson NV, Thomas C, Harrell FE Jr., Wagner D, et al. (1996) The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA 276: 889–897. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC (2008) The performance of different propensity-score methods for estimating relative risks. J Clin Epidemiol 61: 537–545. 10.1016/j.jclinepi.2007.07.011 [DOI] [PubMed] [Google Scholar]
- 24.Ohno I, Hosoya T, Gomi H, Ichida K, Okabe H, Hikita M (2001) Serum uric acid and renal prognosis in patients with IgA nephropathy. Nephron 87: 333–339. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y, Chen W, Jalal D, Li Z, Chen W, Mao H, et al. (2012) Clinical outcome of hyperuricemia in IgA nephropathy: a retrospective cohort study and randomized controlled trial. Kidney Blood Press Res 35: 153–160. 10.1159/000331453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moriyama T, Itabashi M, Takei T, Kataoka H, Sato M, Shimizu A, et al. (2014) High uric acid level is a risk factor for progression of IgA nephropathy with chronic kidney disease stage G3a. J Nephrol. [DOI] [PubMed] [Google Scholar]
- 27.Takiue Y, Hosoyamada M, Kimura M, Saito H (2011) The effect of female hormones upon urate transport systems in the mouse kidney. Nucleosides Nucleotides Nucleic Acids 30: 113–119. 10.1080/15257770.2010.551645 [DOI] [PubMed] [Google Scholar]
- 28.Hak AE, Choi HK (2008) Menopause, postmenopausal hormone use and serum uric acid levels in US women—the Third National Health and Nutrition Examination Survey. Arthritis Res Ther 10: R116 10.1186/ar2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ioannou GN, Boyko EJ (2013) Effects of menopause and hormone replacement therapy on the associations of hyperuricemia with mortality. Atherosclerosis 226: 220–227. 10.1016/j.atherosclerosis.2012.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sciacqua A, Perticone M, Tassone EJ, Cimellaro A, Miceli S, Maio R, et al. (2015) Uric acid is an independent predictor of cardiovascular events in post-menopausal women. Int J Cardiol 197: 271–275. 10.1016/j.ijcard.2015.06.069 [DOI] [PubMed] [Google Scholar]
- 31.Kohagura K, Kochi M, Miyagi T, Kinjyo T, Maehara Y, Nagahama K, et al. (2013) An association between uric acid levels and renal arteriolopathy in chronic kidney disease: a biopsy-based study. Hypertens Res 36: 43–49. 10.1038/hr.2012.135 [DOI] [PubMed] [Google Scholar]
- 32.Sandhu S, Wiebe N, Fried LF, Tonelli M (2006) Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol 17: 2006–2016. [DOI] [PubMed] [Google Scholar]
- 33.Strippoli GF, Navaneethan SD, Johnson DW, Perkovic V, Pellegrini F, Nicolucci A, et al. (2008) Effects of statins in patients with chronic kidney disease: meta-analysis and meta-regression of randomised controlled trials. BMJ 336: 645–651. 10.1136/bmj.39472.580984.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanbay M, Segal M, Afsar B, Kang DH, Rodriguez-Iturbe B, Johnson RJ (2013) The role of uric acid in the pathogenesis of human cardiovascular disease. Heart 99: 759–766. 10.1136/heartjnl-2012-302535 [DOI] [PubMed] [Google Scholar]
- 35.Nagasawa Y, Hasuike Y, Nanami M, Kuragano T, Nakanishi T (2015) Albuminuria and hypertension: the chicken or the egg? Hypertens Res 38: 8–10. 10.1038/hr.2014.135 [DOI] [PubMed] [Google Scholar]
- 36.Zhou J, Chen Y, Liu Y, Shi S, Li X, Wang S, et al. (2014) Plasma uric acid level indicates tubular interstitial leisions at early stage of IgA nephropathy. BMC Nephrol 15: 11 10.1186/1471-2369-15-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koning SH, Gansevoort RT, Mukamal KJ, Rimm EB, Bakker SJ, Joosten MM, et al. (2015) Alcohol consumption is inversely associated with the risk of developing chronic kidney disease. Kidney Int 87: 1009–1016. 10.1038/ki.2014.414 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Osaka University Institutional Ethics Committee (rinri@hp-crc.med.osaka-u.ac.jp, Osaka University Hospital, Future medical Center, Division of clinical Trial, Yamadaoka2-2, Suita, Osaka, Japan).