Abstract
Objective:
To inform initiatives to reduce overuse, we compared neuroimaging appropriateness in a large Medicare cohort with a Department of Veterans Affairs (VA) cohort.
Methods:
Separate retrospective cohorts were established in Medicare and in VA for headache and neuropathy from 2004 to 2011. The Medicare cohorts included all patients enrolled in the Health and Retirement Study (HRS) with linked Medicare claims (HRS-Medicare; n = 1,244 for headache and 998 for neuropathy). The VA cohorts included all patients receiving services in the VA (n = 93,755 for headache and 183,642 for neuropathy). Inclusion criteria were age over 65 years and an outpatient visit for incident neuropathy or a primary headache. Neuroimaging use was measured with Current Procedural Terminology codes and potential overuse was defined using published criteria for use with administrative data. Increasingly specific appropriateness criteria excluded nontarget conditions for which neuroimaging may be appropriate.
Results:
For both peripheral neuropathy and headache, potentially inappropriate imaging was more common in HRS-Medicare compared with the VA. Forty-nine percentage of all headache patients received neuroimaging in HRS-Medicare compared with 22.1% in the VA (p < 0.001) and differences persist when analyzing more specific definitions of overuse. A total of 23.7% of all HRS-Medicare incident neuropathy patients received neuroimaging compared with 9.0% in the VA (p < 0.001), and the difference persisted after excluding nontarget conditions.
Conclusions:
Overuse of neuroimaging is likely less common in the VA than in a Medicare population. Better understanding the reasons for the more selective use of neuroimaging in the VA could help inform future initiatives to reduce overuse of diagnostic testing.
Neuroimaging is common and costly,1 and it is likely that both overuse and underuse exist. Recently, Choosing Wisely guidelines have focused on overuse by questioning the use of diagnostic neuroimaging in 2 highly prevalent neurologic conditions: headache and peripheral neuropathy.2,3 Guideline discordant neuroimaging overuse appears to be common for headache4,5 and peripheral neuropathy.6–8 At the same time, underuse of neuroimaging in some high-risk headache patients9 and diagnostic laboratory testing10 in neuropathy patients likely exists.6
The Veterans Affairs (VA) health system is a single-payer health system that is largely separate from the private sector health care system. Prior comparisons between VA and non-VA systems have suggested that quality of care in the VA is no worse, and often better, than comparable non-VA systems with lower costs.11–14 However, most comparisons have focused exclusively on underuse of medical services.15 The VA system offers a variety of structural and organizational contrasts with the private sector health care system.11,14 Therefore, any differences in inappropriate services between the VA and other health care systems may be partially due to these organizational differences. Consequently, we sought to explore whether rates of inappropriate neuroimaging for headache and neuropathy differs between the VA and a fee-for-service Medicare population. We also examined difference in appropriately performed laboratory studies for the workup of neuropathy. We hypothesized that less overuse and underuse would exist in the VA and that differences in overuse would be more pronounced as more specific definitions of overuse were applied.
METHODS
We performed a retrospective, cross-sectional comparison of the use of diagnostic testing for 2 neurologic conditions (headache and peripheral neuropathy) in the VA and the fee-for-service Medicare population (Health and Retirement Study [HRS]–Medicare) enrolled in the HRS. Our primary goal was to compare the frequency and appropriateness of diagnostic testing between systems.
Standard protocol approvals, registrations, and patient consents.
The Ann Arbor VA Human Studies Committee approved this study with a waiver of informed consent.
Data.
The VA National Corporate Data Warehouse, which contains information on all outpatient encounters and ICD-9 diagnoses in all VA hospitals in the United States, was used to identify outpatient visits for neuropathy and headache in the VA. Data from Medicare Standard Analytic Files (MedPAR, carrier, outpatient) that were linked to the HRS were used to identify the same diagnoses, comorbidities, and care setting. Datasets were limited to individuals age 66 and above for headache (to allow time for Medicare enrollment) and 67 and above for neuropathy (to verify a 2-year neuropathy-free period), and to 2004–2011.
Definition of target conditions.
We identified headache visits in both datasets using the Healthcare Cost and Utilization Program Clinical Classification System16 definition of headache identified at an outpatient visit as the primary diagnosis, ICD-9-CM codes 339.xx, 784.0x, 346.xx, and 307.81. We selected visits with a primary headache diagnosis to maximize specificity of the headache diagnosis and did not exclude patients who had prior headache visits because headache neuroimaging is not necessarily obtained at the initial headache visit. Headache visits identified in either the inpatient or emergency department settings were excluded.
Neuropathy visits were identified with ICD-9-CM codes (354.5, 356.0–9, and 357.0–9) in any diagnosis position in individuals with no neuropathy diagnosis within the prior 24 months.6 We selected visits for patients who had no prior diagnosis of neuropathy (incident) in the previous 24 months because appropriate testing should occur around the time that a new diagnosis is made. We included both primary and all secondary diagnoses because testing should occur when a new diagnosis of neuropathy is made regardless of whether this is the main reason for the visit. Population characteristics including demographics and comorbidities were abstracted from claims to ensure comparability between populations. This approach is known to relatively undercount comorbidities in patients who obtain care in both systems, but particularly in the VA, because there is a lack of financial incentive for complete comorbidity coding.17
Identification of neuroimaging.
Separate episodes of care were defined around the time of the index visit for both headache (6 months after) and neuropathy (6 months before or after). For headache, neuroimaging was identified with Current Procedural Terminology codes for head CT (70450, 70460, 70470) and MRI brain (70551, 70552, 70553). Imaging that was performed in the inpatient or emergency department setting was excluded. For neuropathy, we included 6 months before and after the diagnosis, because testing commonly occurs before a formal diagnosis is made. The definition of neuroimaging for neuropathy was extended to include MRI of the cervical (72141, 72142, 72156), thoracic (72146, 72147, 72157), and lumbar (72148, 72149, 72158) spine. In addition, neurophysiologic (EMG and nerve conduction studies [NCS]) and laboratory testing (fasting glucose, hemoglobin A1C, glucose tolerance test, B12, serum protein electrophoresis [SPEP]) were identified using previously published methods.6
Definitions of appropriateness/potential overuse.
The appropriateness of headache neuroimaging depends on a variety of relatively uncommon clinical factors—red flags in the history and abnormal findings on neurologic examination.18 As these factors are not reliably included in existing datasets, comparisons of appropriateness across health care systems are necessarily limited. To explore the magnitude of potential overuse of neuroimaging without access to these factors, we used a series of increasingly specific definitions for potential overuse. The magnitude of misclassification using these measures should decrease with more specific definitions of overuse. For example, the yield of significant intracranial findings in unselected headache patients is about 2% but decreases by an order of magnitude in patients with migraine; thus a migraine-focused definition of potential overuse should identify fewer false-positives than a definition based on unselected headache.19
To identify potential overuse of headache neuroimaging, we used 3 definitions of increasing specificity. For the least specific definition, we relied on the prior published definition of Schwartz et al.20 and then used 2 additional definitions with increasingly stringent criteria: definition 1, nontraumatic headache (maximally sensitive, nonspecific): any headache diagnosis excluding posttraumatic ICD-9 codes (339.20–339.22, 339.43); definition 2, excluding nontarget conditions: based on the more specific Schwartz et al.20 definition of potential overuse, which excludes a variety of conditions (either in the year prior or in the 6 months after incident diagnosis) where neuroimaging may be appropriate: (cancer [14xx–208.xx, 230xx–239xx], hemiplegic migraine [346.3.x, 346.6x], giant cell arteritis [446.5], epilepsy [345.xx, 780.3x], cerebrovascular disease including TIA [43xx], head or neck trauma [800xx–804xx, 850xx–854xx, 870xx–873xx, 9590x, 910xx, 920xx–921xx], altered mental status [78097 781xx 7845x], and personal history of stroke/TIA or cancer [V1254 V10xx]).20 We added 2 additional nontarget exclusions to the Schwartz et al. criteria to maximize specificity—multiple sclerosis (340.xx) and dementia (290.0, 290.1x, 290.2x, 290.3, 290.4x, 291.2, 294.1x, 046.1, 331.0, 331.1x, 331.2, 331.82); definition 3: migraine excluding nontarget conditions (maximally specific, insensitive)—all nontarget exclusions from definition 2 with narrowing of the included population to only include migraine headaches (346.xx), as guidelines recommend against routine neuroimaging in this population.21,22
For neuropathy, given that neuroimaging should not be obtained as part of the typical evaluation, our least specific definition of potential overuse (definition 1) included all neuroimaging use in patients with neuropathy. To clarify the overall appropriateness of the diagnostic evaluation, we also measured the use of appropriate laboratory testing (B12, SPEP, and glycemic testing) as well electrophysiologic (EMG, NCS) testing, which is of uncertain appropriateness in an unselected neuropathy population.23 We also applied a more specific definition of potential overuse (definition 2): any neuroimaging in patients with neuropathy after excluding nontarget conditions potentially meriting imaging including dementia (as above), MS/myelopathy/central patterns of weakness (336.x, 340.x, 341.x, 342.x, 344.x), epilepsy (345.x), cervical radiculopathy (723.4), lumbar radiculopathy (724.3, 724.4), and stroke (as above).
Statistical analysis.
Descriptive statistics were used to describe neuroimaging utilization for each condition, measure, and population. Comparisons between the VA and non-VA populations were made using 2-sample, 2-tailed tests of proportions across each condition and measure of potential overuse. Neuropathy measures were separately estimated in the overall population and in the population with and without diabetes. Given the substantial differences in the sex of VA and non-VA populations, we also explored whether differences in neuroimaging exist by sex.
RESULTS
Patient populations.
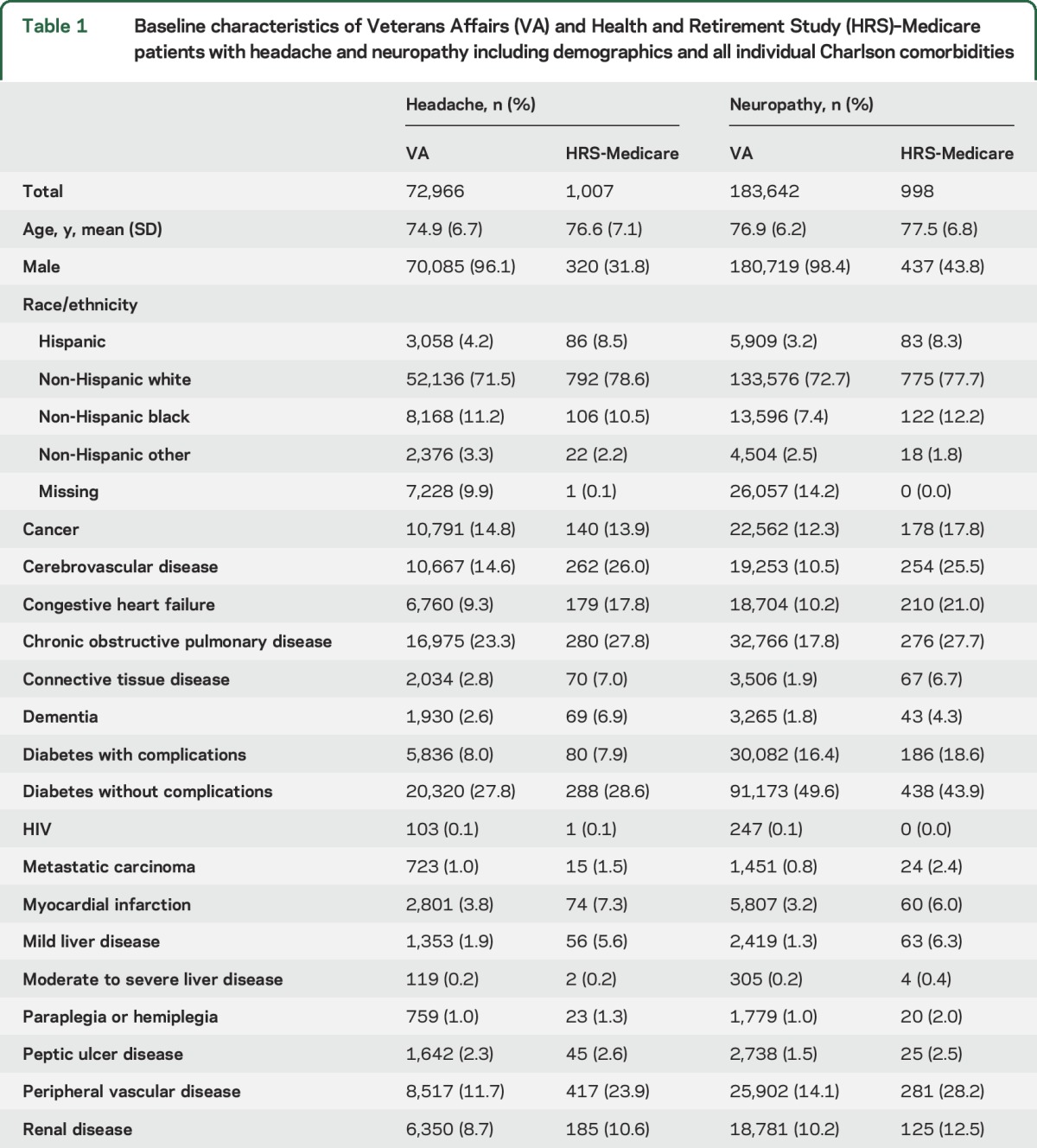
The VA headache population was slightly younger (mean age 75 vs 77 years), generally had fewer coded comorbidities, and, most strikingly, was overwhelmingly composed of men (96% vs 37%) compared with the HRS-Medicare population. Similar patterns were seen in the neuropathy population. Other details of the study populations are summarized in table 1.
Table 1.
Baseline characteristics of Veterans Affairs (VA) and Health and Retirement Study (HRS)–Medicare patients with headache and neuropathy including demographics and all individual Charlson comorbidities
Potential overuse of headache neuroimaging.
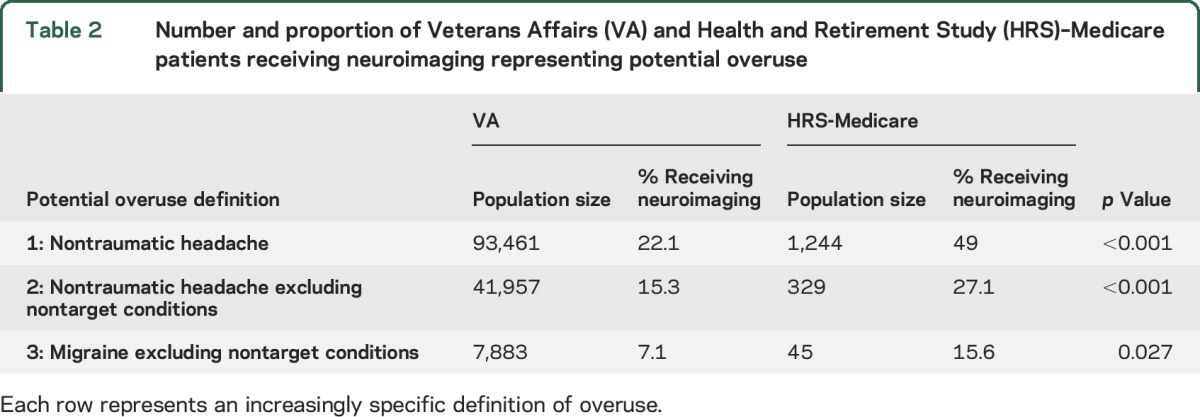
Of the 93,461 VA patients with a primary nontraumatic headache diagnosis (definition 1), 22.1% received neuroimaging, compared with 49.0% of the 1,224 patients in HRS-Medicare (p < 0.001) (table 2). Rates of headache neuroimaging decreased in both systems when looking at more specific definitions of potential overuse. Despite small numbers, a significant difference in imaging rates (8.5% VA vs 18.8% HRS-Medicare, p = 0.04) was observed using the most specific definition of potential overuse. Headache neuroimaging was obtained, on average, 47.1 (SD 47.6) days after the index visit at the VA and 14.2 days (SD 35.3) in HRS-Medicare (p < 0.001). As increasingly specific definitions of potentially inappropriate imaging were applied, the relative rates of neuroimaging in VA and HRS-Medicare remained stable (table 2). There were no differences in neuroimaging by sex in the VA on definition 1 (22.0% in men vs 22.2% in women) or in HRS-Medicare (50.5% in men vs 48.4% in women).
Table 2.
Number and proportion of Veterans Affairs (VA) and Health and Retirement Study (HRS)–Medicare patients receiving neuroimaging representing potential overuse
Potential overuse of neuroimaging in neuropathy.
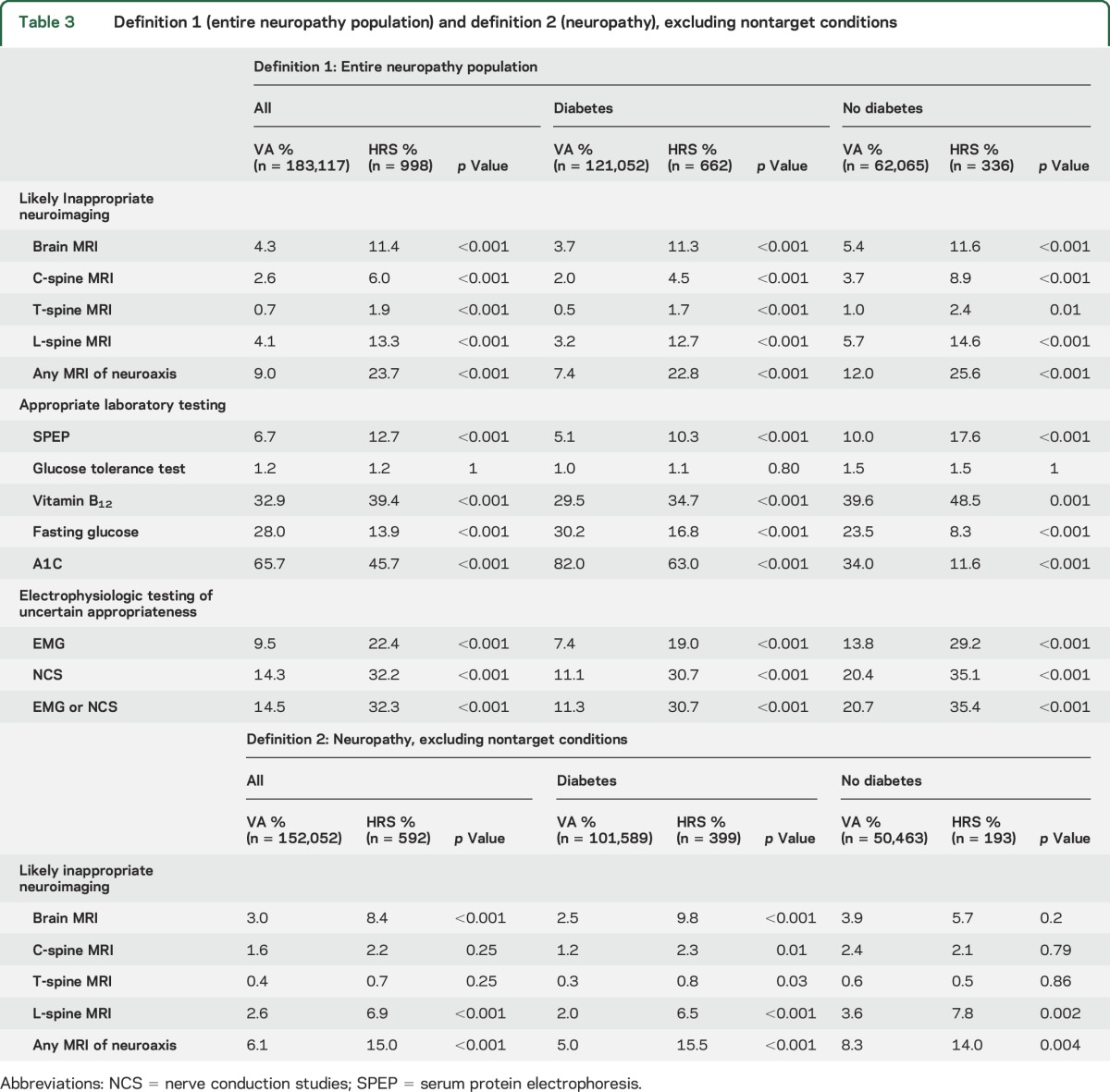
A total 183,117 VA patients and 998 HRS-Medicare patients received a neuropathy diagnosis. On the least specific definition of potential overuse (definition 1, all neuropathy patients), neuroimaging of any component of the neuroaxis was obtained in 9.0% of VA patients vs 23.7% of HRS-Medicare patients (p < 0.001). Appropriate use of laboratory testing varied by setting, with less nonglycemic testing in the VA (SPEP in 6.7% vs 12.7%, p < 0.001, and B12 in 2.9% vs 39.4%, p < 0.001), but more glycemic testing (fasting glucose in 28.0% vs 13.9%, p < 0.001, and hemoglobin A1c in 65.7% vs 45.7%, p < 0.001). Glucose tolerance tests were ordered rarely in both populations (1.2% in both). Lower utilization of neurophysiologic testing, which is of uncertain appropriateness in an unselected population, was also seen at the VA (14.5% vs 32.3% received EMG or NCS, p < 0.001). Similar trends were observed in patients with and without diabetes, although neuroimaging was less common and glycemic testing more common in patients with diabetes (table 3). On the more specific definition of potential overuse, after excluding nontarget conditions (definition 2), imaging rates decreased in both VA and HRS-Medicare, but use of neuroimaging in VA remained significantly lower than in HRS-Medicare (6.1% vs 15.0%, p < 0.001).
Table 3.
Definition 1 (entire neuropathy population) and definition 2 (neuropathy), excluding nontarget conditions
DISCUSSION
For patients with common neurologic diagnoses, we found that potential overuse of neuroimaging was much less common for patients treated in the VA compared with those receiving care through Medicare coverage. While a number of explanations exist, this result suggests that differences in these health care environments may mediate differential use of neuroimaging. Importantly, neuroimaging overuse appears to be high in both the VA and HRS-Medicare populations, indicating that substantial room for improvement exists in both systems.
To meaningfully measure and promote high-quality care, it is important not to reward solely underuse or overuse.24,25 While prior performance measures may have incentivized overuse,26 unnecessary care reduction initiatives, such as Choosing Wisely,27 do the opposite. Without detailed clinical data, it is difficult to know whether the VA's lower headache neuroimaging utilization reflects a global reduction of imaging regardless of indication or a specific reduction in inappropriate utilization. We hypothesized that the difference in neuroimaging utilization between the VA and HRS-Medicare would increase with more specific definitions of potential overuse, but this was not the case. One possible explanation is that VA headache patients had incomplete listings of comorbid diagnostic codes, thereby overstating the degree of inappropriate imaging in the VA. Another possibility is that the number of migraine cases in the HRS-Medicare sample was so small that our analysis may have failed to detect a true difference. Finally, VA headache patients may receive fewer inappropriate imaging tests, but also may have fewer appropriate imaging tests. Further studies with detailed clinical information are needed to definitely determine if the VA promotes less overuse or less testing in general in headache patients.
For neuropathy, we were able to measure not only potential imaging overuse, but also appropriate use of other elements of the neuropathy evaluation. Similar to headache neuroimaging, neuropathy neuroimaging was lower in the VA compared with HRS-Medicare. However, while the VA had higher rates of screening for diabetes, B12 and SPEPs were obtained less commonly in the VA. Screening for diabetes among obese patients is encouraged by VA guidelines,28 so VA's diabetes screening rate may partially reflect diabetes testing not related to neuropathy. So, if one defines quality as limiting both underuse and overuse, neither system performed optimally for neuropathy workup. In addition, even after excluding diagnoses that may justify neuroimaging, potential overuse of neuroimaging was relatively common even in the VA, where 6.1% of patients received a neuroimaging study. These results indicate that the VA system does not necessarily promote less testing across the board, but that neither health care system provides optimal care.
Understanding the factors that mediate the appropriateness of testing between these 2 large health care systems has the potential to inform future efficiency initiatives. Many differences between the VA and Medicare exist and are potential contributory factors: the fixed resource VA environment, the centrality of primary care in the VA,4 use of electronic medical records in the VA,29 provider practice patterns,30 and provider financial incentives.31 Understanding which, if any, of these hypotheses account for the differences in appropriateness may inform future quality improvement initiatives.
While VA patients appear to have less inappropriate imaging than Medicare patients, significant overuse occurs in both systems. Despite extensive literature and guidelines recommending against unnecessary imaging, a significant proportion of headache and neuropathy patients are still receiving these tests. Interventions to curb overutilization are needed in both VA and Medicare. As more and more Veterans receive care in the community due to the Choice Act, it will become even more important to ensure that promoting access to necessary care does not also result in an increase of inappropriate testing.
This study has a number of potential limitations. First, in the absence of detailed clinical data, conclusions about the appropriateness of care can only be tentatively advanced. Second, the VA patient population differs from the HRS-Medicare population in readily measured (i.e., sex) and likely in unmeasured ways. Sex differences are unlikely to explain the lower neuroimaging rates in the VA because neuroimaging rates by sex were similar in both settings. It is plausible that clinically unmeasured factors meriting neuroimaging (red flags) may differ between settings. However, if such differences exist, it is unlikely that they would account for the neuroimaging differences between settings as red flags are not strong predictors of receiving neuroimaging.4 Second, the diagnostic reliability of our ICD-9 algorithms may differ between the VA and HRS-Medicare settings.32,33 Reassuringly, the primary limitation of these definitions is imperfect sensitivity as opposed to limited specificity.33 As VA coding practices also tend toward lower sensitivity,17 this means it is possible that we have understated the difference in neuroimaging utilization between VA and Medicare settings. Third, some VA patients may also be receiving care outside of the VA, and to the extent that VA providers knew the results, they may have refrained from repeating tests. Finally, our Medicare population is limited to patients with fee-for-service Medicare and excludes Medicare Advantage patients. While the financial incentive in capitated Medicare Advantage should lead to less use of imaging, imaging utilization has comparably slowed both in private plans with radiology benefit management strategies34,35 and in Medicare fee-for-service36 in recent years, suggesting this is unlikely to be a major effect.
Our results suggest that the use of inappropriate neuroimaging for headache and neuropathy is relatively common, but less so in the VA than among HRS-Medicare patients. Appropriate laboratory testing for neuropathy is more common in the VA for diabetes testing and more common in HRS-Medicare for other tests. While both constraints in availability of imaging and lack of financial incentives for test ordering may promote less overuse of neuroimaging in the VA, the reasons for underuse of certain laboratory tests are unknown. Future initiatives to reduce overuse of diagnostic testing should learn from what is working in the VA to limit inappropriate imaging but not lose sight of the imperative to enhance the overall quality of care by also motivating appropriate use of needed services.
GLOSSARY
- HRS
Health and Retirement Study
- ICD-9
International Classification of Diseases–9
- NCS
nerve conduction studies
- SPEP
serum protein electrophoresis
- VA
Veterans Affairs
AUTHOR CONTRIBUTIONS
J.F.B. helped design the study, interpreted data, drafted the manuscript, and performed part of the statistical analysis. E.A.K. conceived of the study, aided in data acquisition, critically revised the manuscript, obtained funding, and provided administrative support and supervision. R.J.M. helped design the study, acquired and analyzed data, critically revised the manuscript, and performed statistical analyses. R.H. helped design the study, acquired and analyzed data, critically revised the manuscript, and performed statistical analyses. K.M.L. helped design the study, interpreted data, critically revised the manuscript, and obtained funding and provided administrative support. B.C.C. helped design the study, interpreted data, critically revised the manuscript, and provided technical support.
STUDY FUNDING
The HRS is sponsored by the National Institute on Aging (U01 AG009740) and performed at the Institute for Social Research, University of Michigan. This study was supported in part by the Veterans Health Administration's Office of Informatics and Analytics.
DISCLOSURE
Dr. Burke is funded by NIH grants K08 NS082597 (NINDS) and R01 MD008879 (NIMHD) and has received payments from Astra Zeneca for case adjudication in the SOCRATES trial and for reviewing legal case materials. Dr. Kerr receives research support from the VA, NIH, the Donaghue Foundation, and the Robert Wood Johnson Foundation. She has served on an advisory panel for the Patient Centered Outcomes Research Institute (PCORi). R.J. McCammon and R. Holleman report no disclosures relevant to the manuscript. Dr. Langa was funded by NIH grants AG009740, AG061125, AG018418, AG030155, HS021681, and HS018334, and Veterans Affairs grant HX001276. Dr. Callaghan is supported by the Taubman Medical Institute and NIH K23 grant (NS079417). Dr. Callaghan receives research support from Impeto Medical Inc. He performs medical consultations for Advance Medical and consults for a PCORI grant. This study was supported in part by the Veterans Health Administration's Office of Informatics and Analytics. The opinions expressed are the authors and do not represent those of the US Department of Veterans Affairs or the University of Michigan. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Burke JF, Skolarus LE, Callaghan BC, Kerber KA. Choosing Wisely: highest-cost tests in outpatient neurology. Ann Neurol 2013;73:679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.AANEM's top five Choosing Wisely recommendations. Muscle Nerve 2015;51:617–619. [DOI] [PubMed] [Google Scholar]
- 3.Loder E, Weizenbaum E, Frishberg B, Silberstein S; American Headache Society Choosing Wisely Task Force. Choosing Wisely in headache medicine: the American Headache Society's list of five things physicians and patients should question. Headache 2013;53:1651–1659. [DOI] [PubMed] [Google Scholar]
- 4.Callaghan BC, Kerber KA, Pace RJ, Skolarus L, Cooper W, Burke JF. Headache neuroimaging: routine testing when guidelines recommend against them. Cephalalgia 2015;35:1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callaghan BC, Kerber KA, Pace RJ, Skolarus LE, Burke JF. Headaches and neuroimaging: high utilization and costs despite guidelines. JAMA Intern Med 2014;174:819–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaghan B, McCammon R, Kerber K, Xu X, Langa KM, Feldman E. Tests and expenditures in the initial evaluation of peripheral neuropathy. Arch Intern Med 2012;172:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callaghan BC, Kerber KA, Lisabeth LL, et al. . Role of neurologists and diagnostic tests on the management of distal symmetric polyneuropathy. JAMA Neurol 2014;71:1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callaghan BC. The evaluation of distal symmetric polyneuropathy. Arch Neurol 2012;69:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loder EW, Sheftell F. The quality of headache treatment in the United States: review and analysis of recent data. Headache 2005;45:939–946. [DOI] [PubMed] [Google Scholar]
- 10.England JD, Gronseth GS, Franklin G, et al. . Practice parameter: evaluation of distal symmetric polyneuropathy: role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review): report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology 2009;72:177–184. [DOI] [PubMed] [Google Scholar]
- 11.Jha AK, Perlin JB, Kizer KW, Dudley RA. Effect of the transformation of the veterans affairs health care system on the quality of care. N Engl J Med 2003;348:2218–2227. [DOI] [PubMed] [Google Scholar]
- 12.Shekelle PG, Asch S, Glassman P, Matula S, Trivedi A, Miake-Lye I. Comparison of quality of care in VA and non-VA settings: a systematic review. Washington, DC: Department of Veterans Affairs; 2010. [PubMed] [Google Scholar]
- 13.Asch SM, McGlynn EA, Hogan MM, et al. . Comparison of quality of care for patients in the Veterans Health Administration and patients in a national sample. Ann Intern Med 2004;141:938–945. [DOI] [PubMed] [Google Scholar]
- 14.Trivedi AN, Matula S, Miake-Lye I, Glassman PA, Shekelle P, Asch S. Systematic review: comparison of the quality of medical care in Veterans Affairs and non-Veterans Affairs settings. Med Care 2011;49:76–88. [DOI] [PubMed] [Google Scholar]
- 15.Kerr EA, Chen J, Sussman JB, Klamerus ML, Nallamothu BK. Stress testing before low-risk surgery: so many recommendations, so little overuse. JAMA Intern Med 2015;175:645–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agency for Healthcare Research and Quality (AHRQ). In: HCUP Clinical Classification Software (CCS) for ICD-9-CM [online]. Available at: www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed January 24, 2013. [Google Scholar]
- 17.Byrne MM, Kuebeler M, Pietz K, Petersen LA. Effect of using information from only one system for dually eligible health care users. Med Care 2006;44:768–773. [DOI] [PubMed] [Google Scholar]
- 18.Goadsby PJ. To scan or not to scan in headache. BMJ 2004;329:469–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: the utility of neuroimaging in the evaluation of headache in patients with normal neurologic examinations (summary statement): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 1994:1353–1354. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz AL, Landon BE, Elshaug AG, Chernew ME, McWilliams JM. Measuring low-value care in Medicare. JAMA Intern Med 2014;174:1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000:754–762. [DOI] [PubMed] [Google Scholar]
- 22.Sandrini G, Friberg L, Coppola G, et al. . Neurophysiological tests and neuroimaging procedures in non-acute headache (2nd edition). Eur J Neurol 2010;18:373–381. [DOI] [PubMed] [Google Scholar]
- 23.England JD, Gronseth GS, Franklin G, et al. . Practice parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review): report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology 2009:185–192. [DOI] [PubMed] [Google Scholar]
- 24.Kerr EA, Hayward RA. Patient-centered performance management: enhancing value patients health care systems. JAMA 2013;310:137–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayward RA, Kent DM. 6 EZ steps improving your performance (or how to make P4P pay 4U!). JAMA 2008;300:255–256. [DOI] [PubMed] [Google Scholar]
- 26.Pogach L, Aron D. The other side of quality improvement in diabetes for seniors: a proposal for an overtreatment glycemic measure. Arch Intern Med 2012;172:1510–1512. [DOI] [PubMed] [Google Scholar]
- 27.Choosing Wisely. About The Campaign [online]. Available at: choosingwisely.org/wp-content/uploads/2012/11/choosing-wisely-one-pager.pdf. Accessed July 22, 2013. [Google Scholar]
- 28.The Management of Overweight and Obesity Working Group. Screening and Management of Overweight and Obesity [online] 2014:1–20. Available at: www.healthquality.va.gov/guidelines/CD/obesity/OBESUMC20150106.pdf. Accessed October 8, 2015. [Google Scholar]
- 29.Lammers EJ, Adler-Milstein J, Kocher KE. Does health information exchange reduce redundant imaging? Evidence from emergency departments. Med Care 2014;52:227–234. [DOI] [PubMed] [Google Scholar]
- 30.Cutler D, Skinner J, Stern AD, Wennberg D. Physician Beliefs, and Patient Preferences: A New Look at Regional Variation in Health Care Spending [online]. Cambridge, MA: National Bureau of Economic Research; 2013:1–50. Avaliable at: www.nber.org/papers/w19320. Accessed January 4, 2016. [Google Scholar]
- 31.Baker LC. Acquisition of MRI equipment by doctors drives up imaging use and spending. Health Aff 2010;29:2252–2259. [DOI] [PubMed] [Google Scholar]
- 32.Marrie RA, Yu BN, Leung S, et al. . The utility of administrative data for surveillance of comorbidity in multiple sclerosis: a validation study. Neuroepidemiology 2013;40:85–92. [DOI] [PubMed] [Google Scholar]
- 33.Kolodner K, Lipton RB, Lafata JE, et al. . Pharmacy and medical claims data identified migraine sufferers with high specificity but modest sensitivity. J Clin Epidemiol 2004;57:962–972. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell JM, LaGalia RR. Controlling the escalating use of advanced imaging: the role of radiology benefit management programs. Med Care Res Rev 2009;66:339–351. [DOI] [PubMed] [Google Scholar]
- 35.Levin DC, Bree RL, Rao VM, Johnson J. A prior authorization program of a radiology benefits management company and how it has affected utilization of advanced diagnostic imaging. J Am Coll Radiol 2010;7:33–38. [DOI] [PubMed] [Google Scholar]
- 36.Levin DC, Rao VM, Parker L, Frangos AJ. The sharp reductions in Medicare payments for noninvasive diagnostic imaging in recent years: will they satisfy the federal policymakers? J Am Coll Radiol 2012;9:643–647. [DOI] [PubMed] [Google Scholar]


