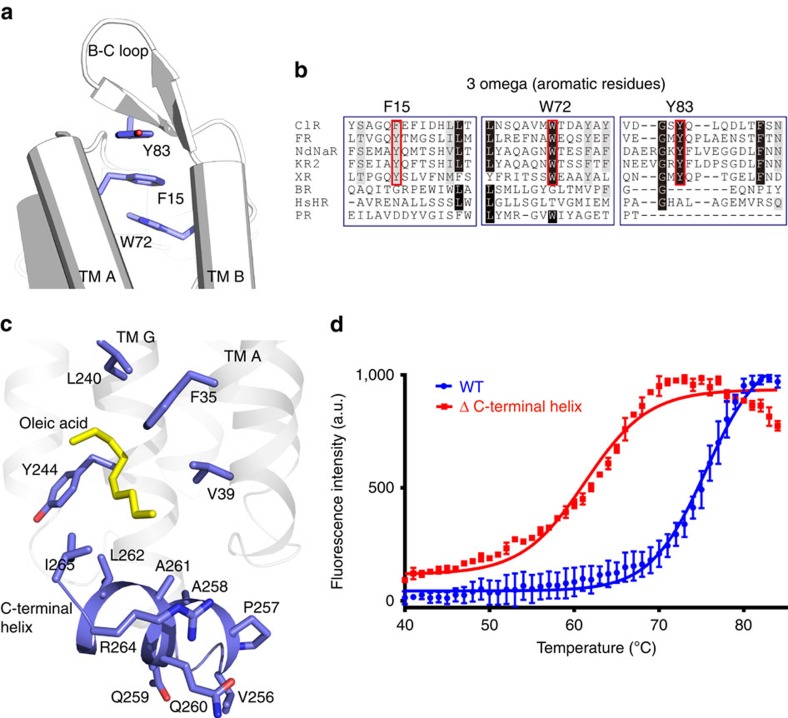
Figure 2. Structural features of ClR.
(a) 3 omega motif of ClR formed by π-stacking interactions of the side chains of aromatic residues in TM A (F15), TM B (W72) and B–C loop (Y83). (b) Sequence alignment around the 3 omega motif. Conserved aromatic residues forming the 3 omega motif are indicated with red open rectangles. ClRs from N. marinus and Fulvimarina pelagi (FR), sodium-pumping rhodopsins from Nonlabens dokdonensis (NdNaR) and Dokdonia eikasta (Krokinobacter eikastus) (KR2), xanthorhodopsin (XR), BR, HR and proteorhodopsin (PR). Identical and similar residues are highlighted in black and grey, respectively. (c) Interaction between the C-terminal helix (blue) and oleic acid (yellow). Hydrophobic residues in TMs A and G, which interact with the oleic acid are also depicted in blue. (d) Fluorescence thermal stability profiles of wild-type (WT) and ΔC-terminal helix ClRs. Tm values are 75 °C for WT and 61 °C for ΔC-terminal helix ClR. The curves are representative of three experiments and the error bars represent the mean±s.d. a.u., arbitrary units.