Figure 1.
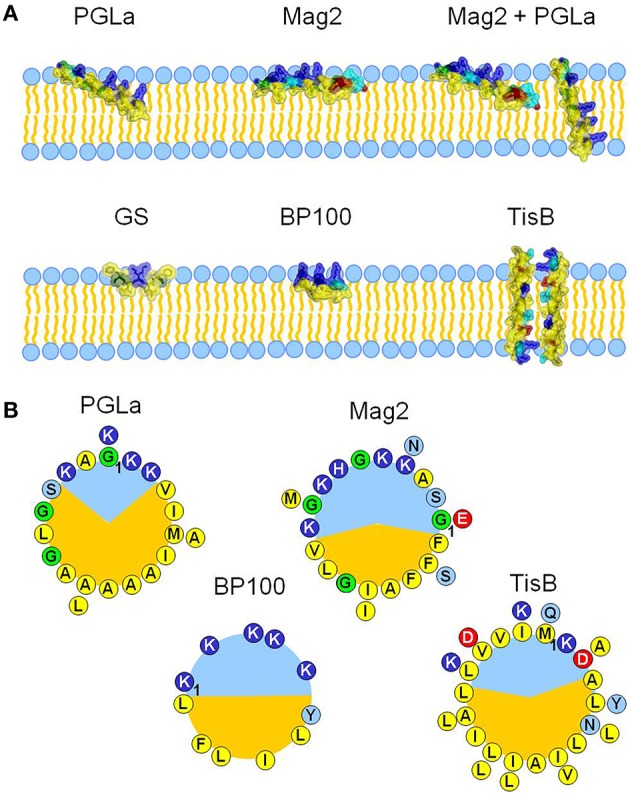
The influence of five different amphiphilic peptides on membrane thickness was examined and compared. All peptides are capable of interacting with cellular membranes, but they represent different structures, sizes and membrane alignments (A), and different amphiphilic profiles (B). PGLa and magainin 2 (Mag2) are α-helical cationic antimicrobial peptides from X. laevis, with lengths of 21 and 23 amino acids, respectively. They adopt an alignment on the surface of the bilayer, but PGLa is able to tilt into the membrane core at high concentrations. It can even insert fully in an upright orientation in the presence of Mag2, with which it forms a synergistically active pair. Gramicidin S (GS) from A. migulanus is a cyclic β-pleated decapeptide, and it aligns preferentially on the membrane surface, just like the α-helical designer-made peptide BP100 (11 amino acids). The amphiphilic α-helical peptide TisB is part of a stress-response system in E. coli and forms a transmembrane dimer. The different alignment states and subsequent interaction with the bilayer are in part a result of the differences in the amphiphilicity of the peptides, which are visualized for all helical peptides as helical wheels (B), with charged residues drawn in red and dark blue, polar residues in light blue, glycines in green, and hydrophobic residues in yellow. PGLa possesses a larger hydrophobic sector (shown in orange) than Mag2 or BP100, correlating with its higher propensity to tilt into the membrane. TisB can dimerize in the membrane via its polar sector.
