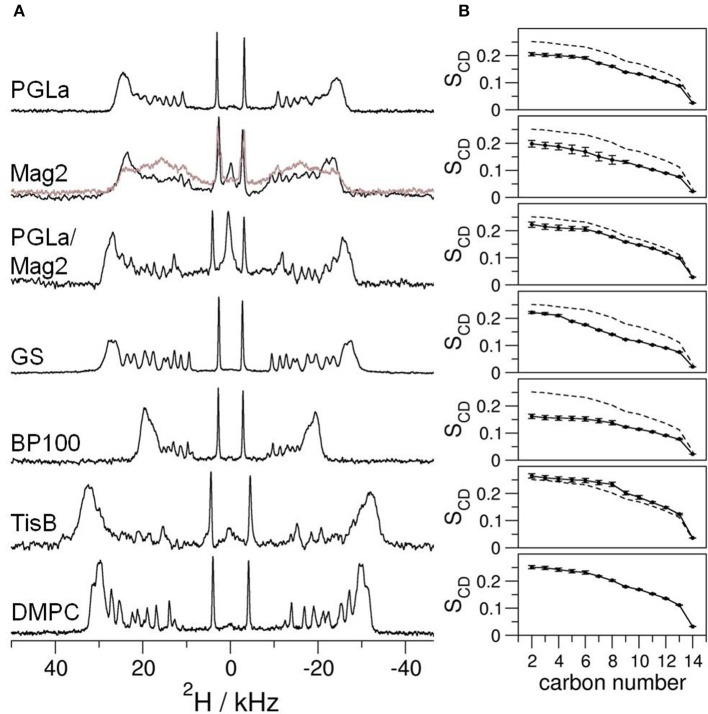
Figure 2.
(A) Solid-state 2H-NMR spectra of oriented bilayers of chain-deuterated DMPC at 32°C. Different peptides as indicated were reconstituted into the membranes at a peptide:lipid ratio of 1:10 (mol/mol). Further peptide:lipid ratios in the range of 1:400–1:20 were studied in the same way (spectra not shown). The bottom 2H-NMR spectrum was obtained from plain DMPC bilayers without peptide. In some of the spectra of Mag2 at the highest peptide:lipid ratio of 1:10 we noticed two components (displayed in gray). (B) To assess the mobility, the order parameter SCD of each deuterated methylene segment was extracted from the splitting of the respective quadrupolar doublet in the corresponding 2H-NMR spectrum. For comparison, the order parameter profile of the plain DMPC sample is shown as dashed line. The error of the order parameters, estimated on grounds of the linewidths of the 2H-NMR spectra, is indicated by error bars.