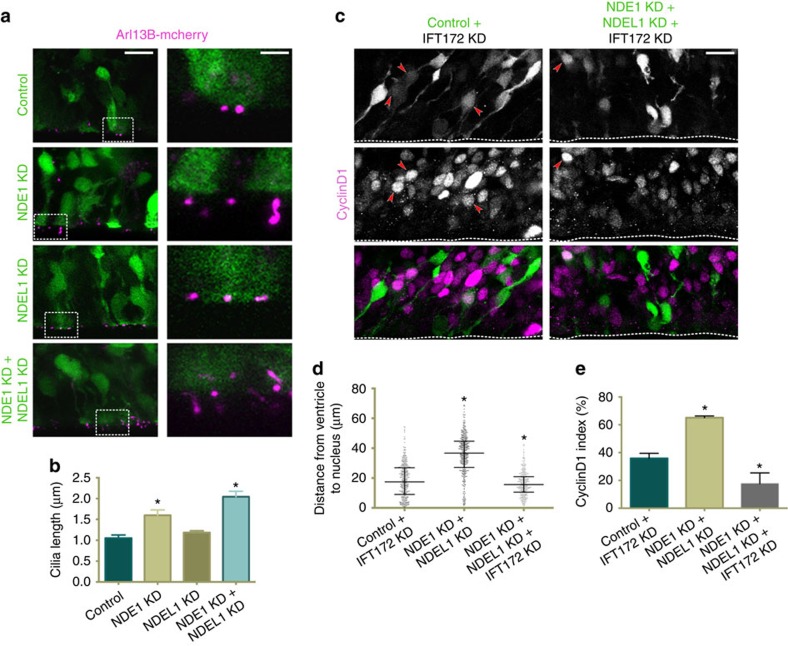
Figure 4. The G1-to-S arrest of radial glia progenitors in the NDE1/NDEL1 double knockdowns disrupts the regulation of primary cilia length.
E16 rat embryonic brains were electroporated with the ciliary membrane marker Arl13B and shRNAs to the various conditions described below. All analyses were done at E19. (a,b) NDE1 knockdown resulted in a significant increase in primary cilia length among electroporated radial glia progenitors (RGPs), though the NDE1/NDEL1 knockdown caused an even greater doubling of primary cilia length. NDEL1 knockdown resulted in no change from control RGP cilia length. (c–e) Inhibition of primary cilia assembly by knockdown of the intraflagellar transport protein IFT172 rescued the CyclinD1 accumulation seen in the NDE1/NDEL1 double knockdown (e). The distribution of apical process lengths of the NDE1/NDEL1/IFT172 triple knockdown more closely mirrored the NDE1 knockdown alone (Fig. 2c). Arrowheads mark electroporated RGP cells positive for CyclinD1. Dashed line indicates ventricle surface. Data are presented as mean±s.e.m. Unpaired t-tests used to compare conditions in b and e, Kolmogorov–Smirnov test used for non-parametric distributions in d, *P<0.05, n=3 embryonic brains from different mothers. Scale bar, 10 μm in a and c, and 2.5 μm in insert panels for a. Also see Supplementary Figs 4 and 5.