Figure 1.
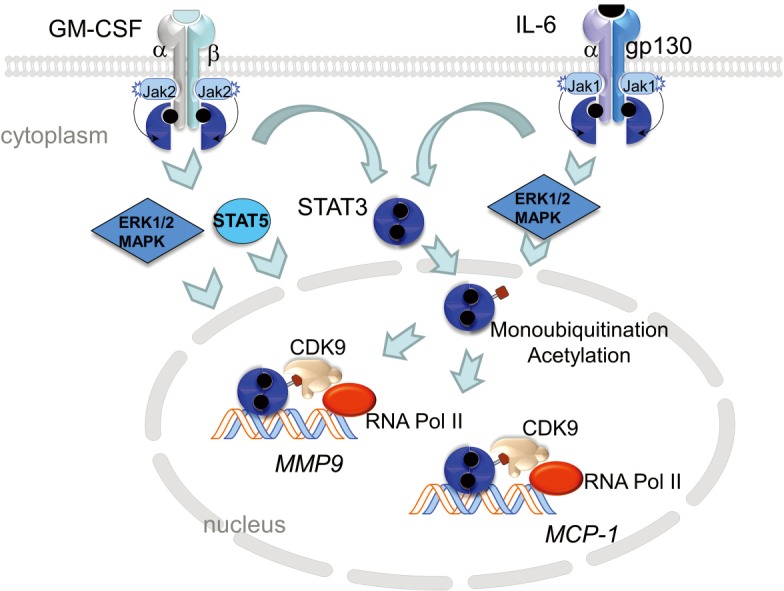
Model of GM-CSF and IL-6 signaling. GM-CSF binding to its receptor, a heterodimer receptor consisting of low-affinity α chain and a longer, signal-transducing β chain leads to conformation changes in the receptor complex and phosphorylation of Janus kinase 2 (JAK2). JAK2 transphosphorylates the β receptor which allows for docking of STAT5 (signal transducer and activator of transcription 5) and STAT3. STAT5 and STAT3 are phosphorylated by JAK2, which promotes their homo-dimerization and translocation to the nucleus to initiate gene transcription. GM-CSFR activation also initiates MAPK signaling involving ERK1/2. Similarly, binding of IL-6 to IL-6Rα leads to dimerization with the signal-transducing membrane protein gp130 and phosphorylation of JAK1. JAK1 transphosphorylates the receptor leading to the recruitment and phosphorylation STAT3. This allows STAT3 to homodimerize and locate to the nucleus. Within the nucleus, phospho-STAT3 is acetylated by p300/CREB-binding protein (CBP) which stabilizes STAT3/p300/CBP complex and promotes enhancesome formation. In addition, STAT3-monoubiquitination on Lys97 promotes binding to BRD4, part of the positive transcription elongation factor (pTEFb) complex that includes CDK9, and promotes transcription elongation of target genes. Both GM-CSF and IL-6 converge on STAT3 signaling suggesting that this common pathway may be a prime target for inhibiting vascular inflammation. GM-CSF, granulocyte macrophage colony-stimulating factor.
