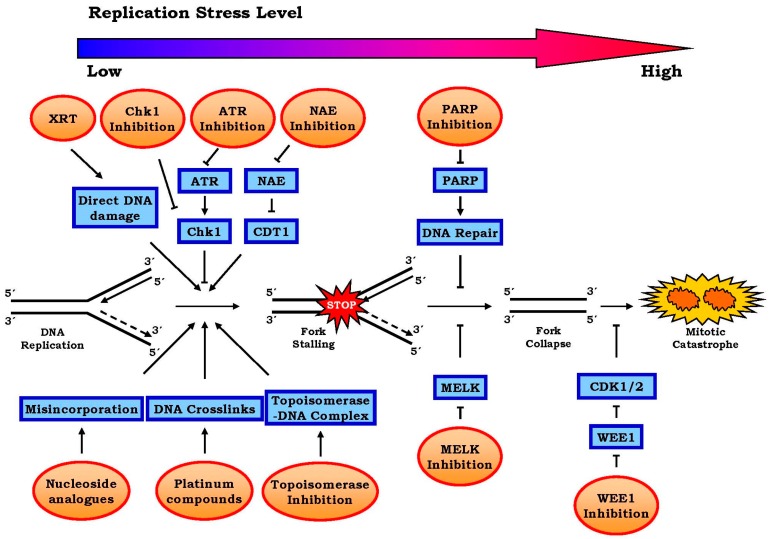
Figure 2.
Illustration of various approaches to target replication stress for cancer treatment. For simplicity, only the approaches that have been discussed in the text are illustrated here. As indicated, with the progression from DNA replication fork stalling to folk collapse and eventually premature entry of mitotic phase, there is accompanying enhancement of DNA replication stress. Shown here are different approaches exploited to enhance this stress level. While chemotherapeutic agents use different approaches to induce fork stalling (e.g., nucleotide disincorporation, DNA crosslinks and topoisomerase—DNA complex), radiation (XRT) induces direct DNA damage. These genetic errors activate ATR-Chk1 signaling which prevents further fork stalling. Therefore, inhibitors of either ATR or Chk1 may enhance replicative stress. Since both PARP and MELK prevent the progression to fork collapse, their corresponding inhibitors may also augment the level of replicative stress. Because the ubiquitin ligase substrate CDT1 causes DNA to replicate more than once and its activity is inhibited by neddylation, the NAE inhibitor can also be used to achieve this purpose. Finally, WEE1 inhibitor activates CDK1/2, therefore facilitating premature entry to S phase. The final consequence of all these approaches is cell death through mitotic catastrophe that is induced by the enhancement of replicative stress.