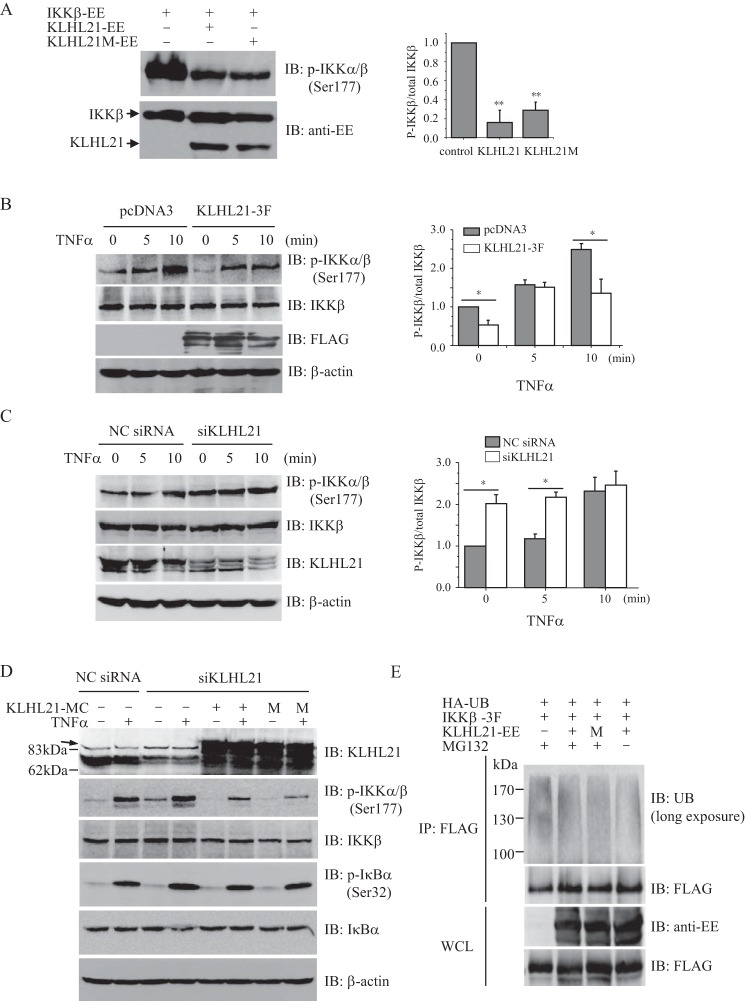
FIGURE 6.
KLHL21 inhibits the activation of IKKβ. A, 293T cells were cotransfected with EE-tagged IKKβ, WT KLHL21, and KLHL21-M mutant, respectively. Phosphorylated IKKβ was analyzed by immunoblotting with anti-phospho-IKKα/β (Ser-177) (left). The density ratio of phosphorylated IKKβ/total IKKβ is shown on the right (n = 3). B and C, 293T cells were transfected with KLHL21–3F (B) and 25 nm siKLHL21 (C), respectively. Phosphorylation of IKKβ in response to TNFα treatment was analyzed by immunoblot with anti-phospho-IKKα/β (Ser-177). The mean results for densitometric scans are shown on the right (n = 3). D, 293T cells were cotransfected with siRNA 3 and mCherry-tagged WT KLHL21 (KLHL21-MC) or KLHL21M mutant (M). 24 h after the transfection, the cells were treated with 20 ng/ml TNFα for 10 min. Phosphorylated and total IKKβ and IκBα were then analyzed by Western blotting. An arrow indicates the nonspecific bands in the KLHL21 immunoblot. E, 293T cells cotransfected with HA-ubiquitin, IKKβ-3F, KLHL21-EE, and KLHL21M-EE were treated with 5 μm MG132 for 16 h. The cells were lysed by boiling in SDS-lysis buffer. The lysates were immunoprecipitated with anti-FLAG and analyzed with the indicated antibodies (long exposure: 10 min). Data are plotted as means ± S.E. (error bars). *, p < 0.05; **, p < 0.01. All experiments were performed three times. IB, immunoblotting; IP, immunoprecipitation.