FIGURE 4.
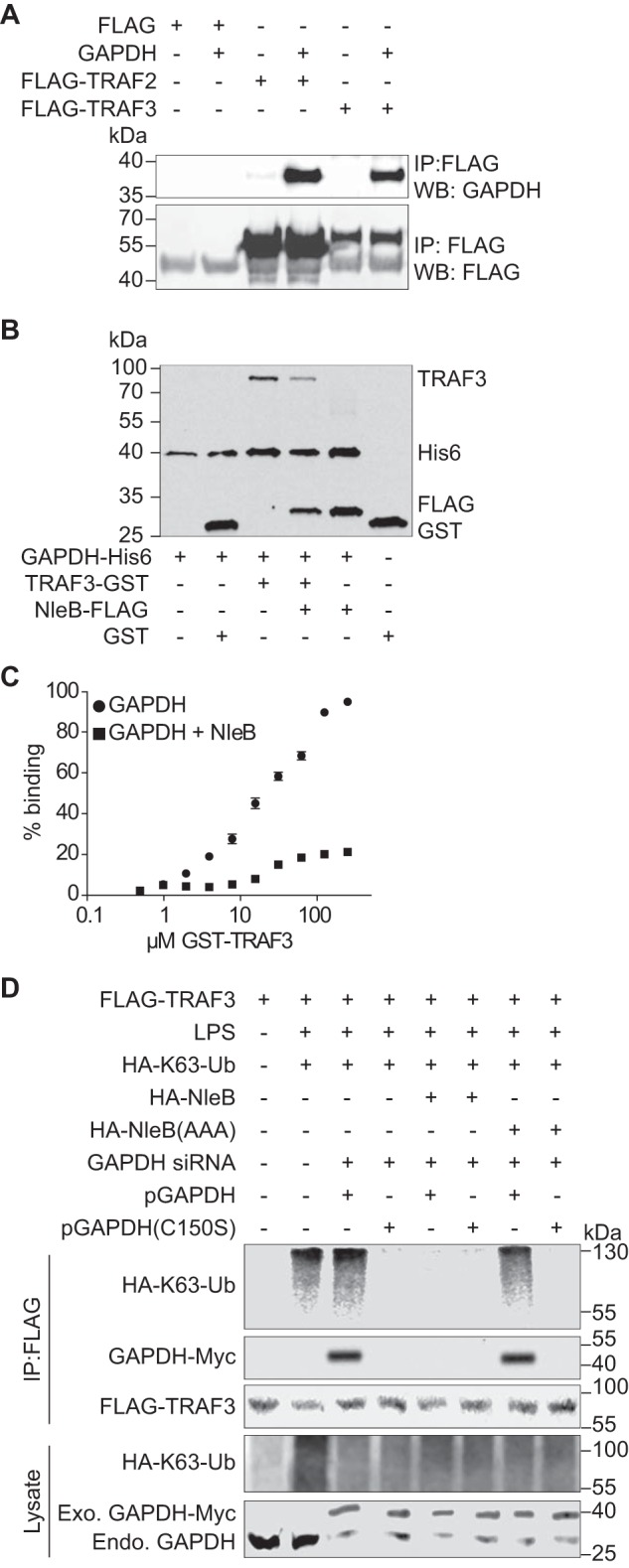
Role of GAPDH in TRAF3 ubiquitination. A, HEK293T cells were co-transfected with Myc-GAPDH and FLAG-TRAF2 or FLAG-TRAF3. After 48 h, cell lysates were immunoprecipitated (IP) with anti-FLAG antibody (Sigma), followed by immunoblotting with anti-GAPDH or anti-FLAG antibody. WB, Western blot. B, His-GAPDH immobilized to nickel-nitrilotriacetic acid-agarose beads were incubated with GST, GST-TRAF3, FLAG-NleB, or GST-TRAF3 + FLAG-NleB. Protein interactions were analyzed using immunoblotting. C, Immulon-2 plates were coated with 1 μg of His-GAPDH and overlaid with GST-TRAF3 in the presence or absence of FLAG-NleB. Protein binding was detected using anti-GST antibody and 1-Step Ultra TMB-ELISA solution. Absorbance at 450 nm was measured. D, HEK-Blue hTLR4 cells were transfected with the indicated plasmids and GAPDH siRNAs. After 72 h, cells were treated with 200 ng/ml LPS for 40 min. Cell lysates were immunoprecipitated using FLAG antibody and immunoblotted for FLAG-TRAF3, HA-K63-ubiquitin, and GAPDH-Myc.
