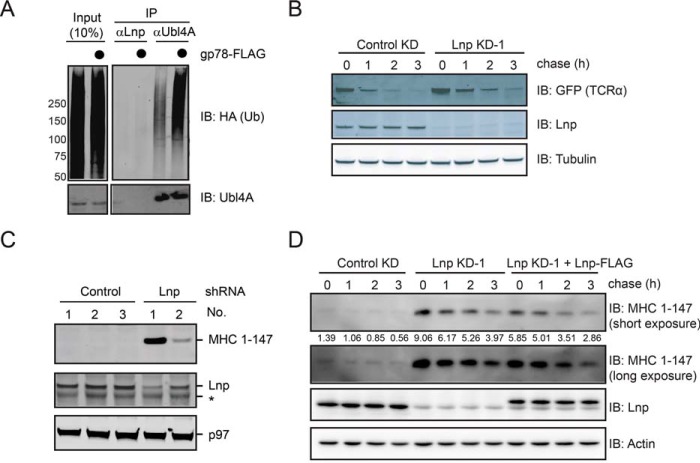
FIGURE 4.
Lnp does not play a significant function in ERAD. A, gp78 does not ubiquitinate Lnp in cells. Endogenous Lnp and Ubl4A were immunoprecipitated (IP) under denaturing conditions from cells transiently transfected with HA-ubiquitin together with either a control or a gp78-expressing plasmid. A fraction of the whole cell extract (input) and the precipitated materials were analyzed by immunoblotting with the indicated antibodies. B, Lunapark knockdown does not significantly affect the degradation rate of TCRα-YFP. The degradation rate of the model ERAD substrate TCRα-YFP in control and Lnp knockdown cells was analyzed by a cycloheximide chase experiment using a cell line stably expressing TCRα-YFP. C, the steady state level of the ERAD substrate MHC(1–147) was examined in cells transfected with three control- and two Lnp-shRNA knockdown plasmids. Whole cell extracts prepared at 48 h post-transfection were analyzed by immunoblotting (IB). Asterisk, a nonspecific band. D, the effect of Lnp knockdown and re-expression on the degradation of MHC(1–147). The degradation of MHC(1–147) in control and Lnp knockdown cells was analyzed by a cycloheximide chase experiment using a cell line transiently expressing FLAG-tagged MHC(1–147). Where indicated, Lnp knockdown shRNA was co-transfected with a Lnp-FLAG construct.