FIGURE 6.
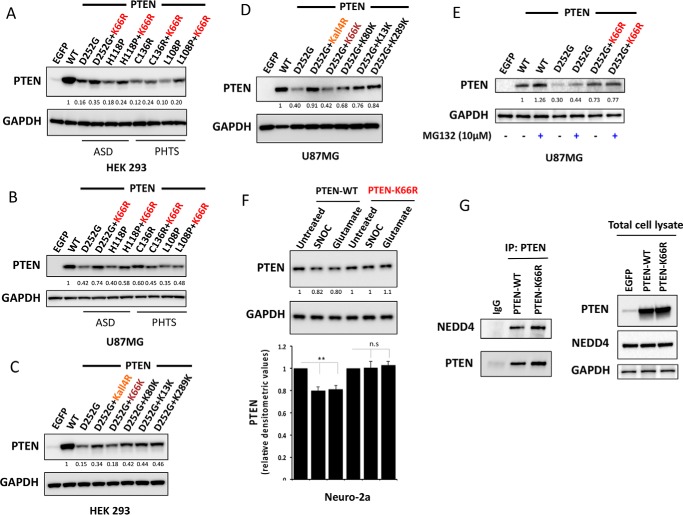
Mutating lysine 66 counteracts destabilizing PTEN mutations associated with autism and tumor syndromes and NO-mediated proteasomal degradation. HEK293T cells (A) and PTEN-null U87MG cells (B) were transfected with vectors encoding PTEN WT or unstable PTEN mutants associated with autism spectrum disorder (ASD) and tumor syndromes (PHTS) or the same unstable mutants carrying an additional K66R mutation. Cells were lysed and PTEN expression was investigated by Western blotting analysis. GAPDH levels were used as a loading control. Representative blots from three independent experiments is shown. Additional mutation of K66R significantly increased the protein expression level of the unstable PTEN mutant proteins D252G, H118P (p value < 0.001), and L108P (p value < 0.01). C and D, effects of PTEN ubiquitination site mutation in PTEN D252G mutant background. HEK293T cells (C) and U87MG cells (D) were transfected as indicated with expressing vectors encoding PTEN WT or PTEN mutants. Cells were lysed and PTEN expression was investigated by Western blotting analysis. GAPDH levels were used as a loading control. A representative blot from three independent experiments is shown. All mutant PTEN proteins containing the K66R mutation showed significant stabilization of D252G mutant enzyme (p value < 0.01). Mutant PTEN D252G-K66K retaining Lys66 in its wild-type form showed no significant effect on D252G mutant enzyme stability. E, U87MG cells transfected with the indicated expression vectors encoding PTEN WT or PTEN mutants were treated for 5 h with 10 μm MG132 proteasome inhibitor before PTEN expression was analyzed by Western blotting analysis for PTEN and GAPDH. F, mouse neuroblastoma Neuro-2a cells were transfected with quantities of the indicated expression vectors encoding PTEN WT or PTEN K66R engineered to lead to similar expression of the two proteins. Cells were treated for 4 h with 200 μm freshly prepared S-nitrosocysteine (SNOC) or 200 μm glutamate before PTEN expression was analyzed by Western blotting for PTEN and GAPDH. A representative blot from three independent experiments is shown (F, lower panel). Bar representing relative densitometric values of PTEN expression after SNOC and glutamate treatments in PTEN-WT and PTEN K66R-transfected cells. The quantitation is derived from three independent experiments and values are mean ± S.E. SNOC acts as a physiological NO donor and glutamate is neurotoxic compound, which induces NO generation. SNOC and glutamate-mediated S-nitrosylation of PTEN has been shown to lead to enhanced PTEN ubiquitination leading to its proteasome-mediated degradation (31). A modest but significant decrease in PTEN levels is observed with SNOC and glutamate treatment in PTEN WT overexpressing cells (two-tailed t test, p value < 0.01). PTEN-K66R does not show this decrease in PTEN levels. G, co-immunoprecipitation of PTEN and NEDD4 in PTEN-WT and PTEN K66R-transfected 293T cells. Quantities of expression vectors encoding PTEN WT or PTEN K66R were engineered to lead to similar expression of the two proteins. PTEN was immunoprecipitated (IP) and PTEN-bound endogenous NEDD4 was immunoblotted.