FIGURE 1.
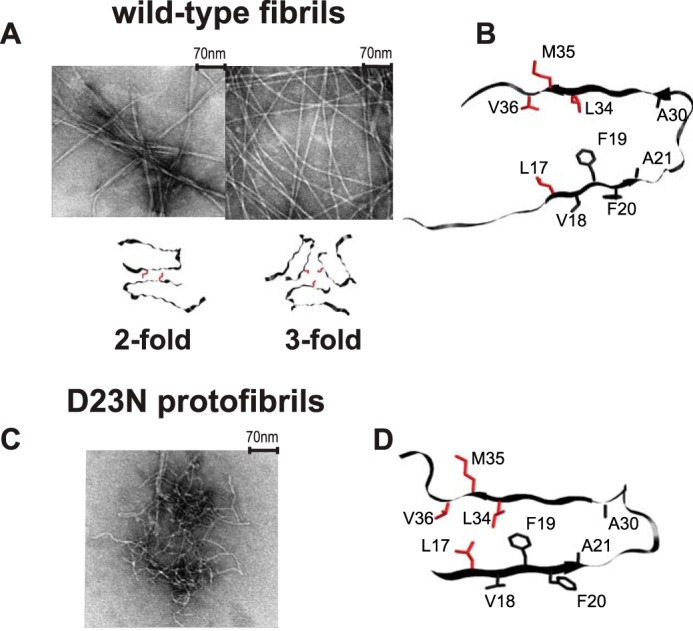
A, examples of transmission electron microscopy images of the wild-type fibrils composed of Aβ1–40 and the corresponding quaternary structures in which the position of Met-35 side chains are color-coded in red. The following polymorphs are shown: striated-ribbon/2-fold symmetric polymorph of native Aβ1–40 (PDB ID 2LMN) and twisted/3-fold symmetric polymorph of native Aβ1–40 (PDB ID 2LMP). B, a ribbon diagram of the monomer corresponding to the wild-type Aβ1–40 in the 2-fold morphology with the side chains of Leu-17, Leu-34, Val-36, and Met-35 investigated in this work shown in red. The 3-fold morphology has a very similar monomeric unit. C, an example of transmission electron microscopy image of the D23N mutant Aβ1–40 protofibrils with antiparallel β-sheet structure. D, a ribbon diagram of the D23N protofibrils with the side chains of Leu-17, Leu-34, Val-36, and Met-35 investigated in this work shown in red (PDB ID 2LNQ).
