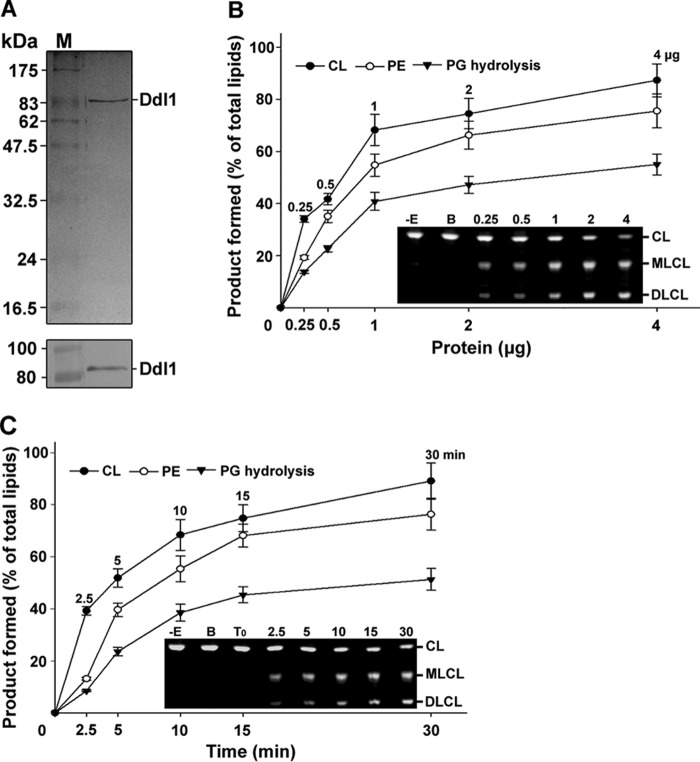
FIGURE 2.
Purification and biochemical function of the Ddl1 protein. A, purification of the recombinant protein. Upper panel, the purified recombinant yeast Ddl1 protein was resolved by 12% SDS-PAGE and stained with Coomassie Brilliant Blue. Lower panel, immunoblot analysis of the Ddl1 protein with an anti-His6 monoclonal antibody (M, protein marker). B, protein-dependent assay. Fluorescently tagged CL, PE, and PG were hydrolyzed with an increasing amount of the purified protein for 10 min at 30 °C (−E, without enzyme; B, boiled enzyme). C, time-dependent assay. The fluorescently tagged phospholipids were hydrolyzed in the presence of 1 μg of the purified protein at 30 °C at different time intervals. T0, zero time point (the enzyme was added to the reaction mixture, which was immediately stopped). The assay was conducted in the presence of 5 μm fluorescently labeled substrate. The reaction was stopped, and the lipids were resolved on a TLC plate and quantified with GeneTools software. A portion of the representative TLC plate is shown. The values are presented as the mean ± S.E. (n = 3).