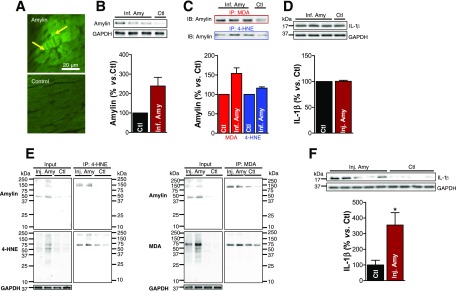
Figure 6.
Langendorff perfusion of mice with aggregated amylin leads to the formation of amylin-4-HNE/MDA adducts and IL-1β activation. A: The 10 μmol/L biotinylated human amylin was recirculated in an isolated mouse heart on a Langendorff apparatus for 2 h. Fluorescence imaging shows binding of fluorescein isothiocyanate-avidin to the circulated biotinylated human amylin, thus demonstrating amylin deposition (arrows) in the heart. B–D: Isolated mouse hearts were perfused with 10 μmol/L aggregated human amylin (n = 3; Inf. Amy) or Tyrode solution (n = 1; Ctl) for 2 h on a Langendorff system, followed by a 10-min washout. B: Western blot measurement of amylin incorporated in the Langendorff perfused hearts. C: 4-HNE and MDA were immunoprecipitated, and the amylin level in the immunoprecipitate was measured by Western blot. D: Western blot analysis of IL-1β in Inf. Amy vs. Ctl hearts. E: C57BL/6 mice were intravenously injected with 2 μg/g body wt aggregated human amylin (n = 4; Inj. Amy) or saline (n = 4; Ctl). Coimmunoprecipitation (IP)/immunoblot (IB) assays of 4-HNE and MDA (left and right panels, respectively) with amylin from amylin-injected and Ctl mice heart homogenates. Input: IB for amylin, 4-HNE or MDA, and GAPDH in the heart homogenates. IP: anti–4-HNE and anti-MDA antibodies pulled down more amylin from amylin-injected compared with Ctl mouse heart homogenates. F: IL-1β level in hearts from mice injected with human amylin vs. Ctl. A representative blot from two independent experiments is shown. Data are presented as the mean ± SE. *P < 0.05.