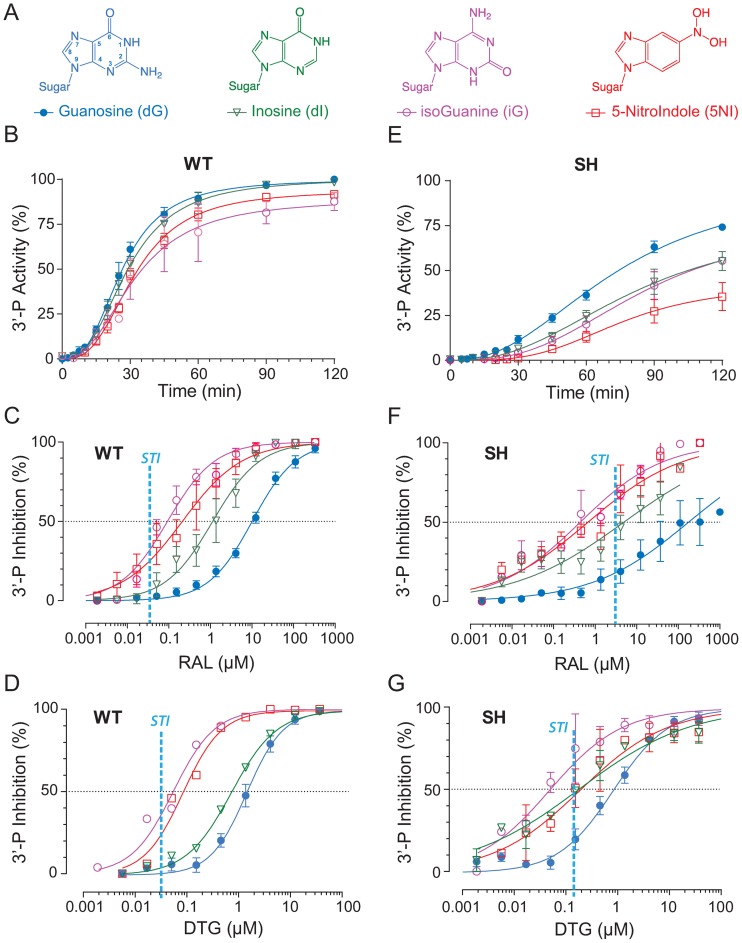
Figure 2.
Effect of +1 nucleobase modifications on IN activity and RAL inhibition. (A) Chemical structure of the modified bases tested. Inosine (dI) and isoguanine (iG) lack the 2-amino group of the guanine base (dG) and iG has flipped substitutions at positions 2 and 6 compared to dG. 5-NitroIndole (5NI) has a nitro substitution at position 5. Time course experiments were used to monitor the 3′-P activity of WT IN (B) or of the SH mutant (D) with the indicated substrates. Using the 120-min time point, inhibition of 3′-P by RAL was determined with the indicated substrates for WT IN (C) and the SH mutant (E). Fit curves and SD were derived from three to five independent experiments. For comparison, the vertical dashed vertical lines represent the IC50 value for RAL and DTG for ST inhibition (STI) for WT IN and the SH mutant.