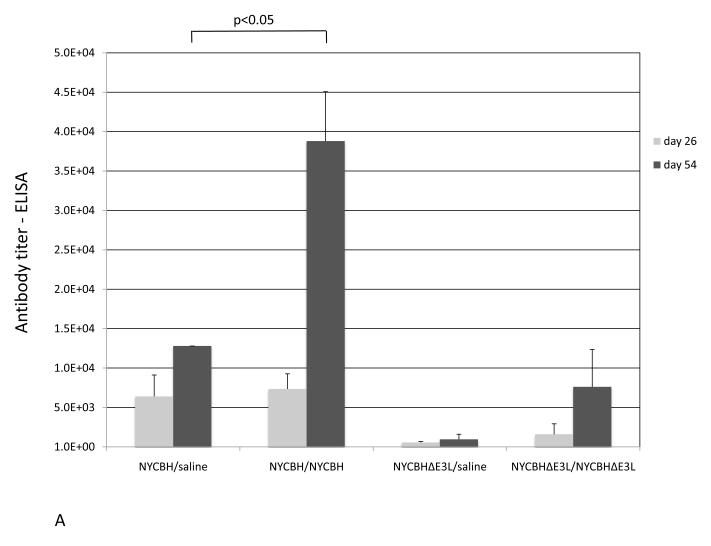
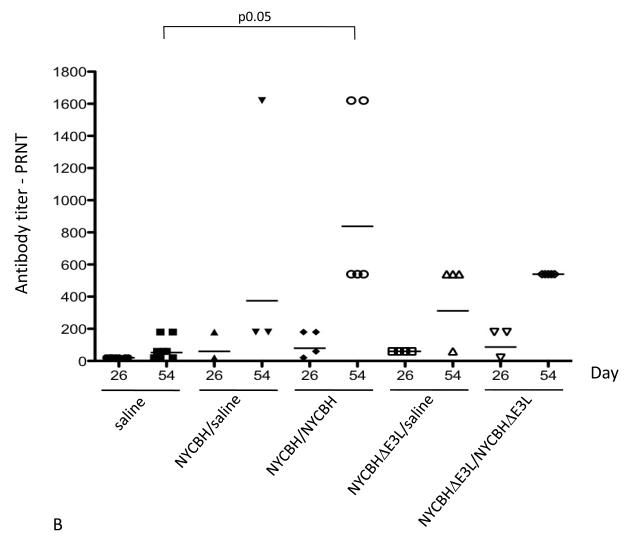
Fig. 4.
Antibody titers in serum following vaccination with NYCBH or NYCBHΔE3L. Groups of 3-5 mice were mock-vaccinated or were vaccinated by scarification with one dose of NYCBH or NYCBHΔE3L on day 0 or with two doses of NYCBH or NYCBHΔE3L on days 0 and 28. Serum samples were harvested prior to challenge at days 26 and 54 from each group of mice and were assayed individually. (A) Geometric means of VACV-specific antibodies were measured by an ELISA. Statistical significance in titers was observed at day 54 between the NYCBH/saline and NYCBH/NYCBH groups, but not between the NYCBHΔE3L/saline and NYCBHΔE3L/NYCBHΔE3L groups. (B) Neutralizing antibodies against ECTV were measured by a plaque reduction neutralization assay (PRNT). Individual PRNTs are shown for each vaccination group, and geometric means are shown as horizontal lines. Statistical significance in titers was observed at day 54 between the saline and NYCBH/NYCBH group only.