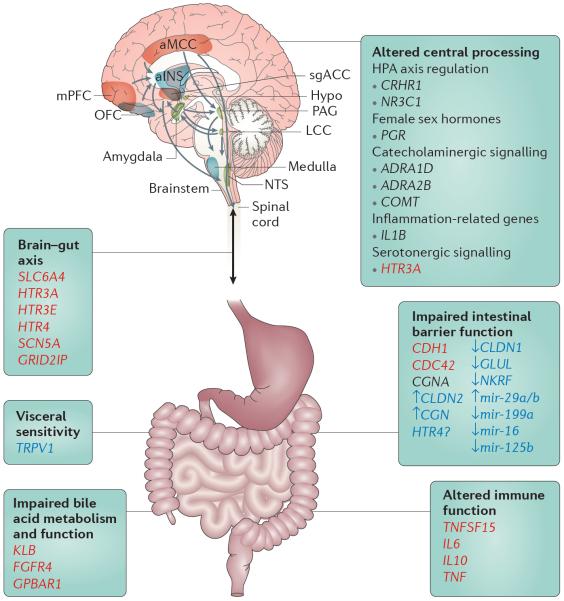
Figure 6. Summary of the genetic findings associated with different pathophysiological mechanisms underlying IBS.
Irritable bowel syndrome (IBS)-related pathways that are potential pharmacogenetic targets are marked in red when based on genetic findings and in blue when based on epigenetic findings; those in black are currently not seen as potential pharmacogenetic targets101. Various pathways might be affected in specific subgroups of patients with IBS: epithelial barrier (permeability), immune function, impaired bile acid metabolism and function, neuronal processing and signal transduction via spinal afferents from the periphery to the central nervous system in addition to the bidirectional crosstalk via the brain–gut axis, presumably contributing to psychological conditions such as anxiety, depression and somatization. Brain networks that have been associated with structural and functional alteration in IBS are depicted. ADRA, adrenoceptor-α; aINS, anterior insula; aMCC, anterior midcingulate cortex; CDC42, cell division cycle 42; CDH1, cadherin 1; CGN, cingulin; CLDN, claudin; COMT, catechol-O-methyltransferase; CRHR1, corticotropin-releasing hormone receptor 1; FGFR4, fibroblast growth factor receptor 4; GLUL, glutamate-ammonia ligase (also known as glutamine synthetase); GPBAR1, G protein-coupled bile acid receptor 1; GRID2IP, GRID2-interacting protein; HPA, hypothalamus–pituitary–adrenal; HTR, 5-hydroxytryptamine receptor; hypo, hypothalamus; IL, interleukin; KLB, Klotho-β; LCC, locus coeruleus complex; mir, microRNA; mPFC, medial prefrontal cortex; NKRF, nuclear factor-κB-repressing factor; NR3C1, nuclear receptor subfamily 3 group C member 1; NTS, solitary nucleus; OFC, orbitofrontal cortex; PAG, periaqueductal grey; PGR, progesterone receptor; SCN5A, sodium voltage-gated channel α-subunit 5; sgACC, subgenual anterior cingulate cortex; SLC6A4, solute carrier family 6 member 4; TNF, tumour necrosis factor; TNFSF15, TNF superfamily member 15; TRPV1, transient receptor potential cation channel subfamily V member 1.