Figure 1.
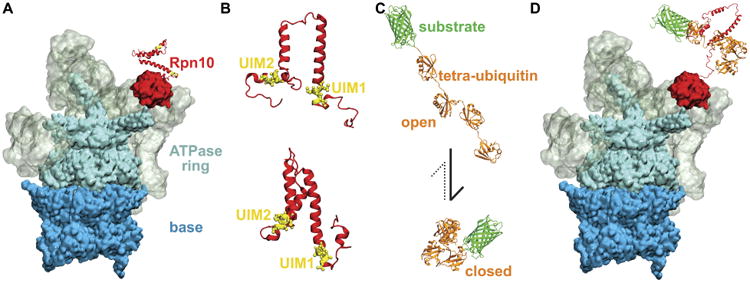
Recognition of K48-linked tetra-ubiquitin by the Rpn10 receptor of 26S proteasome. A. 26S proteasome prior to recognition of the tetra-ubiquitin tag. Different colors mark the main functional components of the proteasome, namely base (blue), ATPase ring (cyan), regulatory particle (grey), and Rpn10 (red). B. Representative structures of Rpn10 flexible arms from NMR experiment (top) and from MD simulations performed in the present study (bottom). UIMs of Rpn10 become protected in MD simulations, while the whole flexible arm becomes more compact than observed in NMR-derived structures. C. K48-linked tetraubiquitin tags (orange) in open and closed forms, covalently bound to an arbitrary substrate (green). The dominant form of K48-linked tetra-ubiquitin in solution is the closed form. D. 26S proteasome after the recognition process, during which the Rpn10 flexible arm and K48-linked tetra-ubiquitin refolded and bound to each other through their hydrophobic interfaces.
