Abstract
Aim
To determine the efficacy of low-dose palliative radiotherapy in patients with refractory aggressive lymphoma.
Background
There are few reports on the administration of palliative radiotherapy to patients with aggressive lymphoma.
Materials and methods
The present study included 11 patients with 30 sites of aggressive lymphoma (diffuse large cell lymphoma, n = 7; mantle cell lymphoma, n = 2; follicular large cell lymphoma, n = 1; and peripheral T cell lymphoma, n = 1). The patients received local palliative radiotherapy after receiving a median of 4 chemotherapy regimens. The radiotherapy doses administered to the 30 sites were as follows: 8 Gy, single fraction (n = 27); 6 Gy, single fraction (n = 1); 4 Gy, single fraction (n = 1); and 4 Gy, 2 fractions (n = 1).
Results
The complete response rate was 45% (5/11); the partial response rate was 36% (4/11). Toxicity occurred at one irradiated site (the mandibular), which showed temporal acute gingivitis; however, medication was not required. Retreatment was required for 3 sites on the head (parotid, face and mandible) due to persistent discomfort. None of the other sites (27/30) required retreatment. A patient with refractory DLBCL underwent radiotherapy (4 Gy, single fraction) for hepatic hilar lymph node involvement but did not recover from jaundice and died of DLBCL.
Conclusions
Eight Gray single fraction radiotherapy was one of meaningful options for the treatment of refractory aggressive lymphoma in terms of its efficacy and the incidence of adverse events. The use of 8 Gy single fraction radiotherapy is therefore recommended for achieving local control in patients with refractory aggressive lymphoma.
Keywords: Low dose radiotherapy, Palliation, Salvage, Refractory lymphoma, Quality of life
1. Background
Non-Hodgkin's lymphomas are heterogeneous group of lymphoproliferative malignancies that are divided into two prognostic groups: indolent lymphomas and aggressive lymphomas.
Many relapses in the initial 2 years after therapy can be salvaged by second- and third-line chemotherapy. However, some patients develop refractory lymphomas that are resistant to all types of chemotherapy and require palliative treatment.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12
Several studies have demonstrated the value of low-dose involved-field radiotherapy (LD-IF-RT) at doses of up to 4 Gy in recurrent follicular lymphoma.1, 2, 3, 5, 6, 7, 9, 10, 11 In contrast, there are few reports on the administration of palliative radiotherapy to patients with aggressive lymphoma 4, 8, 12. Due to the histological nature of both indolent and aggressive lymphomas, their sensitivity to radiation therapy varies.11 Lowry et al. reported that 24 Gy is an effective dose for indolent NHL, while a randomized controlled trial found that the effective dose for aggressive NHL is 30 Gy10 Patients with refractory aggressive lymphoma are treated with more intensive chemotherapy than those with refractory indolent lymphoma.
2. Aim
Recently, 8 Gy single fraction radiotherapy has been applied in the treatment of bone metastasis. Toxicity related to 8 Gy single fraction radiotherapy do not appear to impact the patient's quality of life after treatment. We therefore evaluated the use of 8 Gy single fraction radiotherapy.
3. Materials and methods
We retrospectively analyzed the results of palliative radiotherapy in 11 patients, with a total of 30 sites of refractory aggressive lymphoma, who were treated at our institution between 2002 and 2015. Institutional Review Board approved this study (H19-1). The pathological subtypes were classified according to the World Health Organization (WHO) classification system. The classifications were as follows: follicular lymphoma (FL) grade III (n = 1), mantle cell lymphoma (MCL) (n = 2), peripheral T cell lymphoma (n = 1) and diffuse large B-cell lymphoma (DLBCL) (n = 7).
All of the patients had previously received systemic combination chemotherapy, including salvage regimens. The median number of previous chemotherapy regimens was 4 (range: 2–8).
The patients consulted the Department of Radiation Oncology because they had symptomatic lesions and were diagnosed with incurable disease. The majority of the patients received 8 Gy single fraction radiotherapy. A few of the patients from the early part of the study period were treated with dose escalation.
The planning target volume was defined as the gross target volume with a margin of at least 1 cm in all directions. Fifteen sites were treated with a photon beam and 15 sites were treated with an electron beam. Prophylactic anti-emetics were given to patients who were treated with a wide abdominal radiotherapy field. The response was evaluated at 2–4 weeks after radiotherapy.
The primary endpoint of this study was in-field lymphoma control. Thus, the response definitions of the Revised Response Rate Criteria for Malignant Lymphoma 13 (complete response [CR], partial response [PR], stable disease [SD], and progressive disease [PD]) were applied based on the size of the irradiated lesion. CR required the complete clinical disappearance and/or a normal radiologically detectable size. PR was defined as a ≥50% decrease in diameter. SD was defined as a failure to attain a CR/PR or PD. PD was defined as a ≥50% increase in diameter. The patients were assigned to the most appropriate category during the follow-up period.
Due to the shortness of the remaining life, the time to progression was measured rather than the time to local control (TLC). Toxicity was assessed using the Common Terminology Criteria for Adverse Events v3.0 (CTCAE).
4. Results
A patient with refractory DLBCL received 4 Gy single fraction radiotherapy for hepatic hilar lymph node involvement; however, he did not recover from jaundice and died of DLBCL. Another patient received 6 Gy single fraction radiotherapy for the same condition. He recovered from jaundice and obtained relief from itchiness. Although too small and too heterogeneous in this analysis, we introduced 8 Gy single fraction radiotherapy for patients with refractory aggressive lymphoma.
The patients’ characteristics and clinical courses are shown in Table 1. Among the 11 patients, the overall response rate was 82% (9/11), the CR rate was 45% (5/11), and the PR rate was 36% (4/11). A representative patient in whom a CR was achieved (patient 2 and 11) is shown in Fig. 1, Fig. 2. There was only one case (patient 4) in whom radiotherapy (4 Gy single fraction) failed to achieve a response (SD). The dose was delivered to hepatic hilar lymph node involvement for jaundice; however, the patient did not recover from jaundice and eventually died. Patient 9, who had DLBCL, received 6 Gy (single fraction), which was delivered to the hepatic hilar lymph node to treat jaundice. Patient 9 recovered from jaundice without any adverse side effects. Radiotherapy resulted in good responses in patients with FL (grade III) and MCL.
Table 1.
Patient characteristics and response to palliative radiotherapy for 27 sites in 10 patients.
| Patient | Sex/age (y) | Pathology | Dose | Time since diagnosis (months) | Site | Size (cm) | Response | TLC (month) | Toxicity | Retreatment |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | f/78 | FL(GIII) | 8 Gy ×1 | 123 | Chest wall | 4 | CR | 13 | None | None |
| 8 Gy ×1 | 124 | Axillary | 5 | CR | 12 | None | None | |||
| 2 | m/69 | MCL | 8 Gy ×1 | 86 | Nasal cavity | 5 | CR | 4 | None | None |
| 3 | m/70 | MCL | 8 Gy ×1 | 14 | Abdominal wall | NA | CR | 2 | None | None |
| 8 Gy ×1 | 15 | Neck | NA | Unknown | 1 | None | None | |||
| 8 Gy ×1 | 15 | Abdominal wall | NA | Unknown | 1 | None | None | |||
| 4 | m/65 | PTCL | 8 Gy ×1 | 10 | Neck | 2 | CR | 1 | None | None |
| 8 Gy ×1 | 10 | Skin | NA | CR | 1 | None | None | |||
| 5 | m/38 | DLBCL | 4 Gy ×1 | 44 | Hepatic hilar | 4 | NC | 2 | None | None |
| 6 | m/43 | DLBCL | 2 Gy ×1 | 84 | Chest wall | 4 | PR | 3 | None | None |
| 7 | m/79 | DLBCL | 8 Gy ×1 | 9 | Inguinal | 1 | PR | 4 | None | None |
| 8 | m/73 | DLBCL | 8 Gy ×1 | 9 | Chest wall | NA | CR | 5 | None | None |
| 8 Gy ×1 | 9 | Chest wall | NA | CR | 5 | None | None | |||
| 9 | f/66 | DLBCL | 8 Gy ×1 | 13 | Skull base | NA | PR | 2 | None | None |
| 8 Gy ×1 | 13 | Neck | 6 | PR | 2 | None | None | |||
| 10 | m/70 | DLBCL | 8 Gy ×1 | 46 | Parotid | 6 | CR | 4 | None | 4 months after |
| 8 Gy ×1 | 48 | Face | 3 | PR | 2 | None | 2 months AFTER | |||
| 8 Gy ×1 | 48 | Mandibular | 6 | PR | 2 | a | 2 months after | |||
| 8 Gy ×1 | 50 | Arm | 4 | PR | 4 | None | None | |||
| 8 Gy ×1 | 50 | Wrist | 4 | PR | 4 | None | None | |||
| 8 Gy ×1 | 50 | Ankle | 3 | PR | 4 | None | None | |||
| 8 Gy ×1 | 51 | Leg | 2 | CR | 3 | None | None | |||
| 8 Gy ×1 | 51 | Chest wall | 5 | PR | 3 | None | None | |||
| 8 Gy ×1 | 52 | Hip | 7 | PR | 2 | None | None | |||
| 8 Gy ×1 | 53 | Leg | NA | PR | 1 | None | None | |||
| 8 Gy ×1 | 53 | Leg | NA | Unknown | 1 | None | None | |||
| 6 Gy ×1 | 53 | Hepatic hilar | 6 | PR | 1 | None | None | |||
| 11 | m/87 | DLBCL | 8 Gy ×1 | 46 | Neck | 3 | CR | 4 | None | None |
| 8 Gy ×1 | 46 | Axillary | 4 | CR | 4 | None | None | |||
| 8 Gy ×1 | 46 | Inguinal | 5 | CR | 3 | None | None |
Pathology: FL(GIII): follicular lymphoma (grade III), MCL: mantle cell lymphoma, DLBCL: diffuse large B-cell lymphoma, PTCL: peripheral T-cell lymphoma.
NA: not available TLC: time to local control.
Temporal acute gingivitis.
Fig. 1.
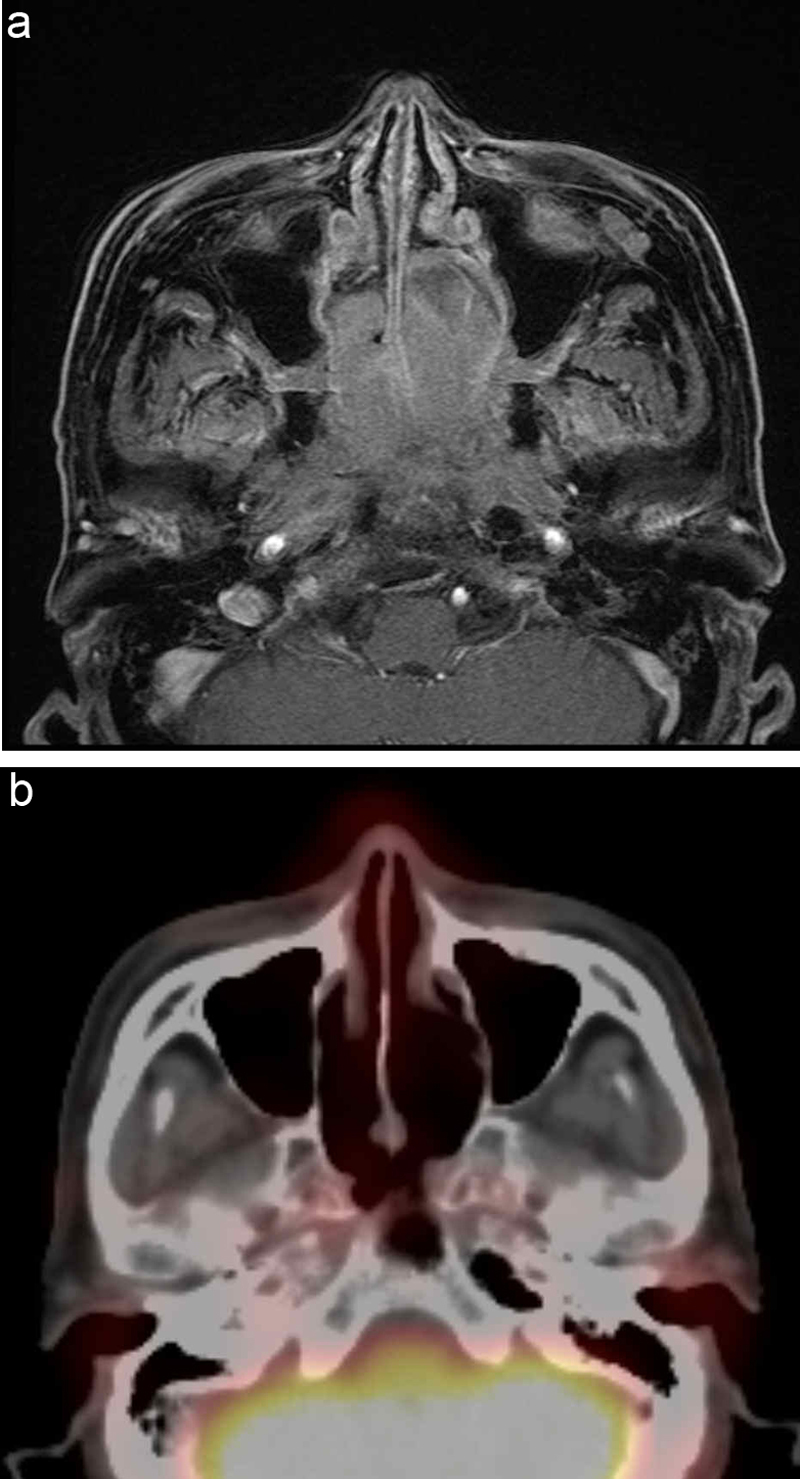
Patient 3, who failed to respond to chemotherapy for mantle cell lymphoma (MCL), presented with nasal bleeding and obstruction at the third progression of MCL and demonstrated nasal airway obstruction due to a bulky mass. (a) Pre-treatment gadolinium-enhanced T1-weighted magnetic resonance imaging (MRI) showing an irregular bulky mass arising from the nasal cavity to the nasopharynx. (b) Post-treatment positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro-d-glucose integrated with computed tomography (18F-FDG PET/CT) showing a complete response at one month after 8 Gy single fraction radiation therapy.
Fig. 2.
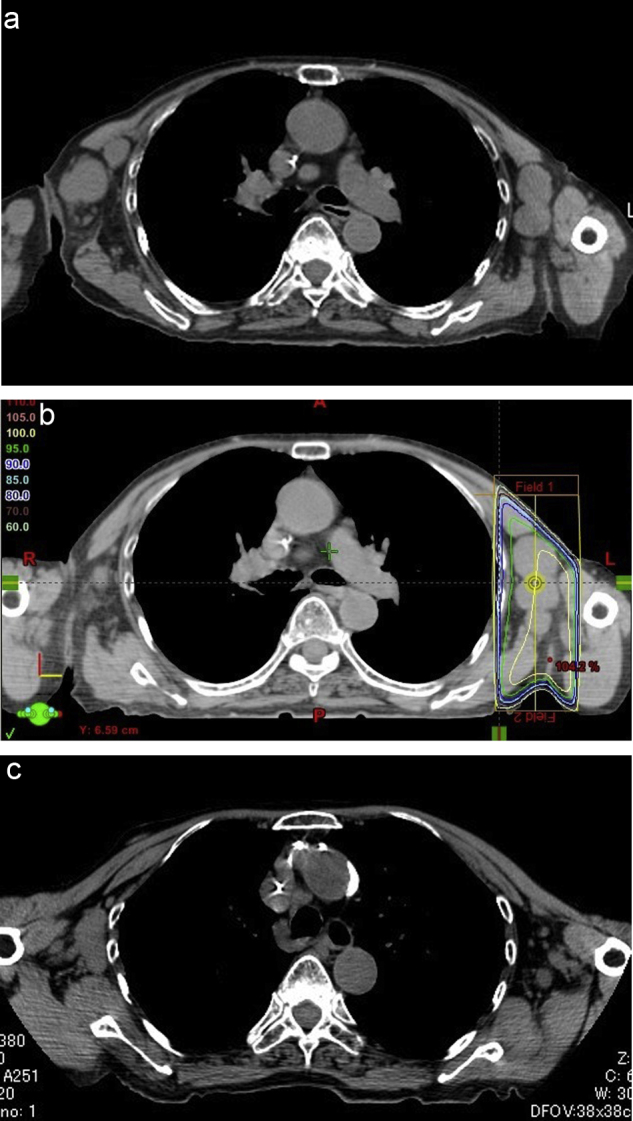
Patient 11, who failed to respond to chemotherapy for diffuse large B-cell lymphoma (DLBCL), presented with pain of the left axillary lymph nodes, which were palpable, at the third progression of DLBCL. (a) Pre-treatment CT showing irregular bulky masses arising from the swollen bilateral axillary lymph nodes. (b) The left axillary lymph nodes were treated with 8 Gy single fraction radiotherapy. The area of the 95% distribution dose (8 Gy) is outlined in green. (c) Post-treatment CT showing a complete response after one month of treatment.
A toxic event was documented at one irradiated site, the mandibular bone in patient 9, which showed temporal acute gingivitis; however, medication was not required and the patient subsequently recovered. Re-irradiation was required for 3 sites on the head (parotid, face and mandible) due to persistent discomfort. No other sites (27/30) required retreatment.
The time to local recurrence (TLR) was short, because the patients were referred to the radiation oncology department in a relapsed, uncontrollable state. The majority of the patients with refractory aggressive lymphoma died due to systemic failure.
5. Discussion
A recent report suggested that a very low-dose RT (4 Gy, 2 fractions) may provide effective local palliation with a negligible rate of toxicity in cases of indolent lymphoma.7, 9, 11 Ganem et al. initially reported the efficacy of low-dose local radiotherapy for palliation.1 Several authors reported similar results regarding the value of low-dose radiotherapy for indolent lymphomas. Haas et al. reported the results of LD-IF-RT in the treatment of 109 indolent lymphomas.6 The OR, CR and PR rates were 92%, 61%, and 31%, respectively. SD and PD were observed in 6% and 2% of the cases, respectively.
However, there is little information regarding the use of palliative radiotherapy in the treatment of aggressive lymphomas. To the best of our knowledge, only a few groups have reported the efficacy of low-dose (4 Gy) palliative radiotherapy in the treatment of refractory aggressive non-Hodgkin's lymphoma.4, 6, 7, 9 Haas et al. reported the differences between indolent and aggressive lymphomas in their response to low-dose palliative radiotherapy. Although this difference was not significant, the response rates in patients with indolent and aggressive lymphomas were 93% and 80%, respectively (p = 0.12), while the CR rates were 56% and 37% (p = 0.08).4 The authors showed the usefulness of low-dose radiotherapy in the treatment of both aggressive lymphomas and indolent lymphomas.
We administered 8 Gy single fraction radiotherapy, as a palliative treatment, to patients with refractory aggressive lymphoma based on our previous experience; irritable jaundice was successfully treated using 6 Gy single fraction radiotherapy, while a patient with a similar condition did not respond to treatment with 4 Gy single fraction radiotherapy. The survival of patients with refractory aggressive lymphoma is limited when chemotherapy is not effective. It is therefore important to relieve their symptoms quickly without adverse events. All of the patients who responded to radiotherapy achieved an improvement in symptoms (e.g., pain or discomfort). However, in the case of palliative care, tumor regression is not an appropriate endpoint. The rate and duration of symptom relief, and the timing in which it is achieved are more important for such patients.
Reducing the need for retreatment to achieve local control is also important. The patient with jaundice who received 6 Gy radiotherapy recovered from jaundice until the end of life. Good effects and recovery from local symptoms were observed in all of the 9 patients who received 8 Gy radiotherapy. Matoba et al. has published two case reports demonstrating the efficacy of 8 Gy single fraction radiotherapy.8
Retreatment was required at three sites in the present study. All three sites were on the face of a patient; he complained of discomfort due to the presence of palpable masses. Each site was treated with palliative 4 Gy single fraction radiotherapy and the patient's discomfort was relieved. Given the above results, it appears that the CR rate for aggressive lymphomas is reasonable, but that retreatment may be required in a limited number of cases. We think these data could potentially lead to questions regarding hypofractionated regimens of higher dose, e.g. 8 Gy 2×, weekly, which are likely to significantly reduce the retreatment rate.
6. Conclusions
Our results showed that 8 Gy single fraction radiotherapy was therefore useful for the treatment of refractory aggressive lymphoma in terms of its efficacy and the occurrence of adverse events. We therefore recommend 8 Gy single fraction radiotherapy to achieve local control in patients with refractory aggressive lymphoma.
Conflict of interest
None declared.
Financial disclosure
None declared.
Acknowledgements
We thank for Dr. Hiroshi Nakamura and Dr. Yuto Hayashi for helping our study.
References
- 1.Ganem G., Lambin P., Socie G. Potential role for low dose limited-field radiation therapy (2 × 2 Grays) in advanced low-grade non-Hodgkin's lymphomas. Hematol Oncol. 1994;12:1–8. doi: 10.1002/hon.2900120102. [DOI] [PubMed] [Google Scholar]
- 2.Sawyer E.J., Timothy A.R. Low dose palliative radiotherapy in low grade non-Hodgkin's lymphoma. Radiother Oncol. 1997;42:49–51. doi: 10.1016/s0167-8140(96)01854-3. [DOI] [PubMed] [Google Scholar]
- 3.Girinsky T., Guillot-Vals D., Koscielny S. A high and sustained response rate in refractory or relapsing low-grade lymphoma masses after low-dose radiation: analysis of predictive parameters of response to treatment. Int J Radiat Oncol Biol Phys. 2001;51:148–155. doi: 10.1016/s0360-3016(01)01626-1. [DOI] [PubMed] [Google Scholar]
- 4.Haas R.L., Poortmans P., de Jong D. High response rates and lasting remissions after low-dose involved field radiotherapy in indolent lymphomas. J Clin Oncol. 2003;21:2474–2480. doi: 10.1200/JCO.2003.09.542. [DOI] [PubMed] [Google Scholar]
- 5.Ng M., Wirth A., Ryan G., MacManus M. Value of low-dose 2 × 2 Gy palliative radiotherapy in advanced low-grade non-Hodgkin's lymphoma. Aust Radiol. 2006;50:222–227. doi: 10.1111/j.1440-1673.2006.01566.x. [DOI] [PubMed] [Google Scholar]
- 6.Haas R.L., Poortmans P., de Jong D. Effective palliation by low dose local radiotherapy for recurrent and/or chemotherapy refractory non-follicular lymphoma patients. Eur J Cancer. 2005;41:1724–1730. doi: 10.1016/j.ejca.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 7.Haas R.L. Low dose radiotherapy in indolent lymphomas, enough is enough. Hematol Oncol. 2009;27:71–81. doi: 10.1002/hon.882. [DOI] [PubMed] [Google Scholar]
- 8.Matoba M., Oota K., Tonami H. Palliative radiotherapy with 1 × 8 Gy using conformal radiotherapy for chemotherapy-refractory, recurrent, aggressive lymphomas. Jpn J Radiol. 2010;28:220–223. doi: 10.1007/s11604-009-0400-x. [DOI] [PubMed] [Google Scholar]
- 9.Chan E.K., Fung S., Gospodarowicz M. Palliation by low-dose local radiation therapy for indolent non-Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2011;81:781–786. doi: 10.1016/j.ijrobp.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Lowry L., Smith P., Qian W. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother Oncol. 2011;100:86–92. doi: 10.1016/j.radonc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Hoskin P.J., Kirkwood A.A., Popova B. 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): a randomised phase 3 non-inferiority trial. Lancet Oncol. 2014;15:457–463. doi: 10.1016/S1470-2045(14)70036-1. [DOI] [PubMed] [Google Scholar]
- 12.Tseng Y.D., Chen Y.H., Catalano P. Rates and durability of response to salvage radiation therapy among patients with refractory or relapsed aggressive non-Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2015;91:223–231. doi: 10.1016/j.ijrobp.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 13.Cheson B.D., Pfistner B., Juweid M.E., Gascoyne R.D., Specht L., Horning S.J., Coiffier B., Fisher R.I., Hagenbeek A., Zucca E., Rosen S.T., Stroobants S., Lister T.A., Hoppe R.T., Dreyling M., Tobinai K., Vose J.M., Connors J.M., Federico M., Diehl V. International Harmonization Project on Lymphoma. J ClinOncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]


