Fig. 1.
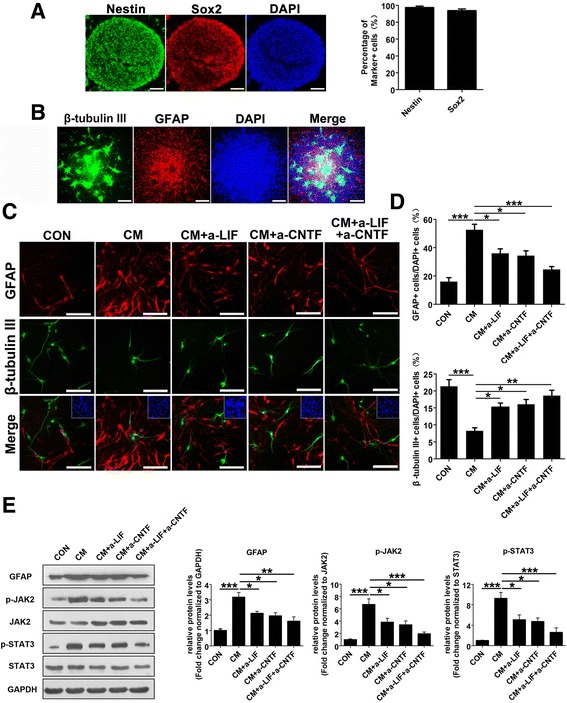
Effects of reactive astrocytes on NSC differentiation. a Immunofluorescence labeling of whole-mount embryonic cortical neurospheres showing the expression of NSC marker nestin (green); DAPI (blue) was used for nuclear staining. Scale bar, 100 μm. b The whole-mount neurospheres allowed to differentiate for 7 days in the absence of bFGF and hEGF were stained with the neuron marker β-tubulin III (green) and astrocyte marker GFAP (red); DAPI (blue) was used for nuclear staining. Scale bar, 200 μm. c The dissociated neurospheres were exposed to DMEM/F12/1 % N2 plus media supplement medium (CON) and reactive astrocyte-conditioned medium alone (CM), with LIF or/and CNTF neutralization antibody (CM + a-LIF, CM + a-CNTF, or CM + a-LIF + a-CNTF) to differentiate for 4 days in the absence of bFGF and hEGF, followed by fluorescence staining with β-tubulin III (green) and GFAP (red). Inset: DAPI staining of cell nuclei in the field of view. Scale bar, 100 μm. d The proportions of GFAP-positive or β-tubulin III-positive cells from the experiment shown in c were determined. Values are means ± SEM. Asterisks indicate a statistically significant difference compared with the CON or CM group (*P < 0.05, **P < 0.01, ***P < 0.001, one-way analysis of variance (ANOVA) followed by Tukey’s test, n = 3 independent experiments). e Western blot analysis and quantifications of p-JAK2, p-STAT3, and GFAP protein expressions in NSCs treated with different conditioned media indicated in c for 4 days. JAK2 and STAT3 were used as internal controls. Values are means ± SEM. Asterisks indicate a statistically significant difference compared with the CON or CM group (*P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA followed by Tukey’s test, n = 4 independent experiments). For quantitative analysis, the numbers of each type of cell scored in four random fields were averaged, utilizing the Image-Pro Plus image analysis software
