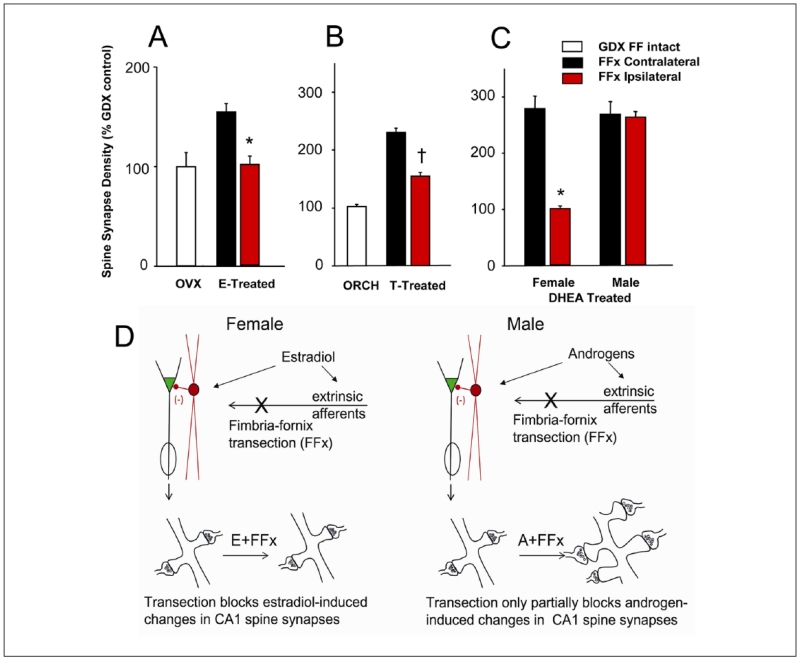
Figure 1.
Effects on synapse formation in response to gonadal steroid administration of unilateral transection of the subcortical afferents to the hippocampus, by cutting the right fimbria/fornix (FF), are dependent on sex, as well as the nature of the gonadal steroid treatment. (A) Morphometric estimation of the density of spine synapses in the ipsilateral (red bars) and contralateral (black bars) CA1 stratum radiatum of unilaterally fimbria/fornix transected female rats. Rats were either ovariectomized (OVX) or OVX and treated with estradiol benzoate (2 × 10 μg, 24 hours apart, s.c.). FF transection had no effect on CA1 synapse density in OVX rats, but completely abolished the increase in synapse density induced by estradiol ipsilateral to the transection. *Significantly different from results on the contralateral side of the brain. (B) Density of pyramidal cell spine synapses in the CA1 stratum radiatum of intact male, orchidectomized (ORCH) and ORCH testosterone (T)-treated rats. Approximately equal spine synapse densities are present in the hippocampi of control males, ORCH T replaced males, and FF-transected T-replaced males contralateral to the FF transection. The spine synapse response to T is partially inhibited ipsilateral to FF transection. *Significantly different from control, intact males; †Significantly different from both intact and ORCH males. (C) Spine synapses in the CA1 stratum radiatum of gonadectomized female and male rats after unilateral and dehydroepiandrosterone (DHEA) treatment. FF transection abolished the synaptic response to DHEA treatment ipsilateral to the transection in female, but not male, rats. In contrast to the results for T replacement, DHEA replacement in males was able to completely restore spine synapse density ipsilateral to FF transection. *Indicates a significant difference between the synapse densities in the ipsilateral and contralateral CA1. (D) Schematic illustration of the possible underlying mechanisms. In females, FF transection completely abolishes the ability of estradiol or the aromatizable androgen DHEA to increase CA1 spine synapse density, whereas in males the effects of androgen are only partially impaired by the elimination of subcortical afferents (adapted from data in Kovacs and others 2003; Leranth and others 2000; Mendell and others 2013).