Figure 1.
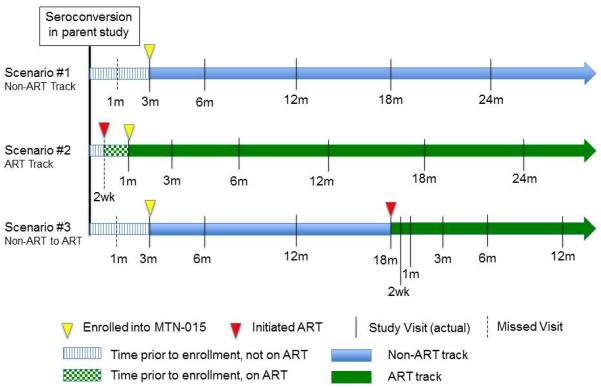
Examples of MTN-015 visit schedules. In Scenario 1, a participant is enrolled 3 months after seroconversion; the month 1 visit is missed. In Scenario 2, a participant begins ART prior to enrollment into MTN-015. The target dates for the visit schedule are based on the ART initiation date. In Scenario 3, a participant is enrolled 3 months after seroconversion and begins ART 18 months after seroconversion. The target dates for subsequent study visits are reset to be based on the ART initiation date.
