Abstract
Background
Patients with cirrhosis and refractory ascites have physiologic and hormonal dysregulation that contributes to decreased kidney function. Placement of a transjugular intrahepatic portosystemic shunt (TIPS) can reverse these changes and potentially improve kidney function. We sought to evaluate change in estimated glomerular filtration rate (eGFR) following TIPS.
Study Design
Retrospective, matched cohort analysis.
Settings & Participants
Patients who underwent first-time TIPS placement for refractory ascites in 1995–2014. Frequency matching was used to generate a comparator group of patients with cirrhosis and ascites treated with serial large-volume paracentesis (LVP) in a 1:1 fashion.
Predictor
TIPS placement compared to serial LVP.
Outcome
Change in eGFR over 90 days’ follow-up.
Measurements
Multivariable regression stratified by baseline eGFR <60 vs. ≥60 mL/min/1.73 m2; analysis of effect modification between TIPS placement and baseline eGFR.
Results
276 subjects (TIPS, n=138; serial LVP, n=138) were analyzed. After 90 days, eGFR increased significantly after TIPS placement in subjects with baseline eGFR <60 mL/min/1.73 m2 compared to treatment with serial LVP (21 [95% CI, 13–29] mL/min/1.73 m2; p <0.001) and was no different in those with eGFR ≥60 mL/min/1.73 m2 (1 [−9 to 12] mL/min/1.73 m2; p = 0.8). There was significant effect modification between TIPS status and baseline eGFR (p = 0.001) in a model that included all subjects.
Limitations
Outcomes restricted by clinically recorded data; clinically important differences may still exist between TIPS and LVP cohorts despite good statistical matching.
Conclusions
TIPS placement was associated with a significant improvement in kidney function. This was most prominent in subjects with baseline eGFR <60 mL/min/1.73 m2. Prospective studies of TIPS in populations with eGFR <60 mL/min/1.73 m2 are needed to evaluate these findings.
Keywords: transjugular intrahepatic portosystemic shunt (TIPS), TIPS placement, large-volume paracentesis (LVP), cirrhosis, refractory ascites, estimated glomerular filtration rate (eGFR), renal function, mortality, portal hypertension, liver, kidney
Patients with decompensated cirrhosis and refractory ascites suffer from high morbidity and mortality.1–3 Treatment is challenging, as medical therapy with diuretic agents often does not prevent ascites formation.1, 4 Many of these patients require serial large-volume paracentesis (LVP) or placement of a transjugular intrahepatic portosystemic shunt (TIPS) as part of their management. Given the heterogeneity in clinical response to TIPS seen in randomized trials, there is often a reluctance to deploy it as first-line therapy for refractory ascites.1, 5–13
The pathophysiology of refractory ascites can offer insight to which patients may benefit most from TIPS placement. Cirrhosis is characterized by portal hypertension and splanchnic vasodilation, which leads to a complicated cascade of pathophysiologic changes, including decreased effective circulating volume/intravascular volume depletion, decreased kidney perfusion, activation of systemic neurohumoral responses, and ultimately a downstream effect of sodium and water retention.14–16 Placements of a TIPS reverses many of these changes by redirecting portal venous blood flow back into the systemic circulation, thereby improving effective circulating volume and alleviating the hormonal cascade responsible for ascites formation.1, 17–21 Hepatorenal syndrome is an example of the extreme of this pathophysiology, where the hemodynamic alterations from portal hypertension lead to kidney injury that exceeds the Acute Kidney Injury Network (AKIN) threshold of a serum creatinine increase of ≥0.3 mg/dL.4, 16, 22–24 However, all patients with refractory ascites may have some degree of reduced kidney function even if they do not meet criteria for hepatorenal syndrome, as small studies have shown improvements in serum creatinine/estimated glomerular filtration rate (eGFR), renin/aldosterone levels, and urine sodium excretion immediately following TIPS placement for refractory ascites.7, 14, 17–21, 25–35
Given the potential link between TIPS placement and kidney function, we sought to better describe the effect of TIPS placement on eGFR. We hypothesized that those with lower baseline eGFR would have greater improvement in kidney function after TIPS placement, given their advanced pathophysiology related to portal hypertension. Despite decreased kidney function leading to a higher Model for End-Stage Liver Disease (MELD) score,36 we believe this improvement in kidney function will help offset the higher expected mortality in this subgroup.
Methods
Patient Population and Data Collection
We performed a retrospective cohort study of all patients who had a first-time TIPS successfully placed for refractory ascites from 1995 through 2014 at two tertiary care centers (Massachusetts General Hospital and Brigham and Women’s Hospital). Data were identified using the Partners Healthcare Research Patient Data Registry, a centralized clinical data warehouse designed for research and quality improvement purposes.37, 38 The TIPS recipients were identified using the ICD-9-CM code “39.1 – intra-abdominal venous shunt” as well as review of the TIPS procedure census from Massachusetts General Hospital. Two authors (A.S.A., G.O.) manually identified all TIPS recipients to confirm the ICD-9-CM code corresponded to a new TIPS placement and the indication of refractory ascites was consistent with guideline definitions.3 Fifty subjects underwent a complete chart review to confirm accuracy of the electronic data registry.
Matched Comparison Group
A comparison cohort of subjects with cirrhosis and refractory ascites (without a history of TIPS placement) who had at least two LVPs in a 90-day period was identified via the data registry using the CPT codes for abdominal paracentesis (49082 and 49083). Paracentesis volume and diuretic dosing were determined by treating clinicians. Albumin was used for volume repletion after paracentesis per local standard of care.3 Using a frequency-matching algorithm, these patients were matched in a 1:1 ratio to the cohort of TIPS recipients by MELD score (grouped as ≤6, 7–12, 13–18, 19–24, and ≥25, with higher score indicating higher predicted 3-month mortality). As there was no reference date analogous to the date of TIPS placement in this cohort, multiple potential 90-day periods existed for each subject. After examination of this distribution, the 90-day period with the most LVPs was selected for each subject in order to best compare to the TIPS cohort.
Definition of Outcomes
The primary outcome in this study was change in eGFR over 90 days (calculated using the CKD-EPI [Chronic Kidney Disease Epidemiology Collaboration] creatinine equation).39 In the TIPS cohort, eGFR was obtained at four time points over a span of 180 days: within 72 hours pre- and post-TIPS placement, the closest value to 90 days pre-TIPS (mean, 23 ± 21 [standard deviation] days from target) and the closest value to 90 days post-TIPS (22 ±21 days from target). In the LVP cohort, eGFR was obtained closest to three time points separated by 90 days: day 0 (day of the first LVP in the study; 3 ±9 days from target), day 90 (13 ±23 days from target), and day 180 (14 ±24 days from target). Values were not included if they were more than 90 days from the target follow-up date. Only one value was captured at each time point to avoid frequency of testing bias, as sicker subjects were likely to have more eGFR values measured around each time point. Subjects who were dialysis dependent prior to TIPS (or day 0 in the LVP group) were excluded from analysis of eGFR. The secondary outcome in this study was death at 90 days. Those subjects who did not have a confirmed vital status at 90 days were excluded from this analysis. Demographics presented in Table 1 were recorded at the time of TIPS placement (or day 0 of follow-up for the LVP cohort). Baseline eGFR was measured immediately before TIPS placement (or closest to day 0 of follow-up for the LVP cohort).
Table 1.
Demographics and baseline characteristics in TIPS and serial LVP cohorts.
| All | TIPS | LVP | P value | |
|---|---|---|---|---|
| (n = 276) | (n = 138) | (n = 138) | ||
| Age (y) | 58 (11) | 58 (11) | 59 (12) | 0.4 |
| Female sex | 90 (33%) | 46 (33%) | 33 (32%) | 0.8 |
| White race | 223 (81%) | 117 (85%) | 106 (77%) | 0.09 |
| Non-Hispanic ethnicity | 257 (93%) | 130 (94%) | 127 (92%) | 0.5 |
| Co-morbidities* | ||||
| Diabetes mellitus | 104 (38%) | 49 (36%) | 55 (40%) | 0.5 |
| Hypertension | 158 (57%) | 59 (43%) | 99 (72%) | <0.001 |
| Coronary artery disease | 62 (23%) | 35 (25%) | 27 (20%) | 0.2 |
| Etiology of cirrhosis | 0.07 | |||
| NASH | 25 (9%) | 16 (12%) | 9 (7%) | |
| Alcohol | 77 (28%) | 42 (30%) | 35 (25%) | |
| HCV infection | 60 (22%) | 28 (20%) | 32 (23%) | |
| Mixed etiology | 36 (13%) | 22 (16%) | 14 (10%) | |
| Other | 78 (28%) | 30 (22%) | 48 (35%) | |
| Listed for liver transplant | 103 (38%) | 53 (40%) | 50 (37%) | 0.6 |
| MELD score | 17 (8) | 17 (8) | 16 (8) | 0.2 |
| No. paracenteses, per 90 d** | 5.5 (3.9) | 5.7 (4.1) | 5.3 (3.7) | 0.3 |
| Prior history of encephalopathy*** | 125 (47%) | 56 (43%) | 69 (50%) | 0.3 |
| Mean arterial pressure (mmHg) | 81 (13) | 79 (11) | 82 (15) | 0.06 |
| On furosemide**** | 199 (76%) | 102 (82%) | 97 (70%) | 0.03 |
| Furosemide dose***** (mg/d) | 56 (32) | 70 (62) | 43 (52) | <0.001 |
| On spironolactone****** | 169 (65%) | 94 (76%) | 75 (54%) | <0.001 |
| Spironolactone dose (mg/d) | 80 (90) | 104 (100) | 59 (74) | <0.001 |
| Laboratory data | ||||
| Sodium (mmol/L) | 134 (5) | 134 (5) | 134 (5) | 0.9 |
| SUN, mg/dL | 31 (22) | 35 (22) | 28 (21) | 0.01 |
| Creatinine (mg/dL) | 1.7 (1.3) | 1.7 (1.0) | 1.6 (1.5) | 0.6 |
| Baseline eGFR (mL/min/1.73 m2) | 60 (32) | 52 (27) | 67 (35) | <0.001 |
| AST (U/L) | 94 (133) | 99 (161) | 90 (96) | 0.6 |
| ALT (U/L) | 57 (92) | 56 (93) | 59 (91) | 0.8 |
| Alkaline phosphatase (U/L) | 178 (201) | 148 (111) | 209 (261) | 0.01 |
| Total bilirubin (mg/dL) | 4.2 (6.2) | 3.4 (5.2) | 5.1 (7.0) | 0.03 |
| Albumin (g/dL) | 2.8 (0.6) | 2.8 (0.6) | 2.8 (0.6) | 0.8 |
| International normalized ratio | 1.5 (0.4) | 1.5 (0.4) | 1.5 (0.3) | 0.3 |
| White blood cells (103/µL) | 7.2 (4.3) | 6.9 (3.8) | 7.4 (4.8) | 0.4 |
| Hemoglobin (g/dL) | 10.3 (1.9) | 10.1 (1.6) | 10.4 (2.1) | 0.3 |
| Platelets (103/µL) | 123 (86) | 115 (75) | 131 (95) | 0.1 |
eGFR (estimated glomerular filtration rate), TIPS (transjugular intrahepatic portosystemic shunt), LVP (large-volume paracentesis), NASH (non-alcoholic steatohepatitis), MELD (Model for End-Stage Liver Disease), AST (aspartate transferase), ALT (alanine transferase). SUN, serum urea nitrogen; HCV hepatitis C virus
Note: Values for categorical variables are given as number (percentage); for continuous variables, as mean ± standard deviation. Baseline values were taken immediately before TIPS placement, or closest available to day 0 of follow-up for the LVP cohort. Conversion factors for units: bilirubin in mg/dL to µmol/L, ×17.1; creatinine in mg/dL to µmol/L, ×88.4; SUN in mg/dL to mmol/L, ×0.357;
Comorbidities were documented by International Classification of Diseases, Ninth Revision, code prior to study enrollment per electronic database review.
Available for 107/138 subjects in the TIPS cohort and all subjects in the LVP cohort.
Available for 268/276 subjects.
Available for 263/276 subjects.
Converted to equivalent dose of furosemide if on another loop diuretic
Available for 262/276 subjects.
Statistical Analysis
An eGFR of 60 mL/min/1.73 m2 was chosen as a clinically important cut-point for stratification given its relevance in CKD staging.40 Analysis of the primary outcome (change in eGFR over 90 days between TIPS and LVP cohorts) was performed using a multivariable linear regression model stratified by eGFR and adjusted for age, etiology of cirrhosis, and baseline eGFR. Analysis of the secondary outcome (death by 90 days) was performed using a multivariable logistic regression model, stratified by eGFR and adjusted for age and baseline eGFR at the time of TIPS (or first LVP). Effect modification between TIPS placement status and baseline eGFR was assessed in both models for the entire cohort. Sensitivity analyses of the primary outcome were performed by (1) liver transplant listing status, (2) for those with an eGFR available at all time points, and (3) using a maximum-likelihood, mixed-effects repeated-measures model for change in eGFR across all time points in both cohorts. All model assumptions were checked and given a normal distribution, and parametric testing was used for all univariate and multivariable analysis. Continuous variables were presented as means ± standard deviation and regression models were presented with 95% confidence intervals (CIs). Descriptive tables were stratified by baseline eGFR and analyzed using Student’s t-tests and Chi square tests. SAS version 9.4 (SAS Institute Inc, Cary, NC) was used for all analyses. Two-tailed p values <0.05 were considered statistically significant.
Ethics Statement
The Partners Institutional Review Board approved this study (protocol number 2015P000357). All procedures and practices abide by the guidelines set forth by the Declaration of Helsinki. The need for informed consent was waived for this study.
Results
General Demographics and Subject Characteristics
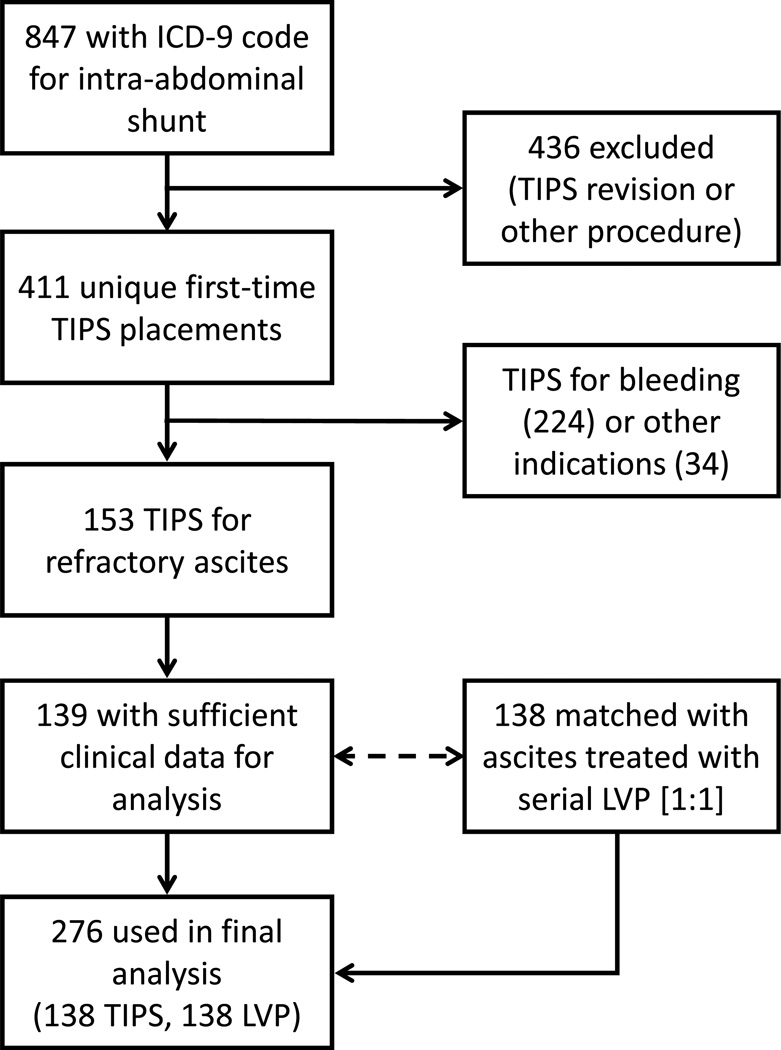
As shown in Table 1 and Figure 1, two hundred seventy six subjects were used in the final analysis. There were ninety-three TIPS subjects and 62 serial LVP subjects with a baseline eGFR <60 mL/min/1.73 m2, leaving 45 TIPS subjects and 76 serial LVP subjects with an eGFR ≥60 mL/min/1.73 m2 (Table 2). In stratified analysis, MELD score was similar in TIPS and LVP cohorts for both strata: mean values of 20 ±8 vs. 20 ±8 [p = 0.6] for eGFR <60 mL/min/1.73 m2 and 12 ±5 vs. 13 ±6 [p = 0.5] for eGFR ≥60 mL/min/1.73 m2. An exact number of paracenteses in the 90 days prior to TIPS was reported in 107 of 138 (78%) of the TIPS cohort, and was similar to the number reported in a 90-day period in the LVP group for both strata (means of 6.3 ±4.6 vs. 5.7 ±3.8 [p = 0.5] for eGFR <60 mL/min/1.73 m2 and 4.9 ±2.9 vs. 4.9 ±3.6 [p = 0.9] for eGFR ≥60 mL/min/1.73 m2). Six subjects (4%) were dialysis-dependent before TIPS, while 19 subjects were dialysis dependent at day 0 of follow up in the LVP cohort (p = 0.01).
Figure 1. Flow diagram of subject selection.
Key: ICD-9 (Internal Classification of Diseases, Ninth Revision), TIPS (transjugular intrahepatic portosystemic shunt), LVP (large-volume paracentesis).
Table 2.
Demographics and baseline characteristics in TIPS and serial LVP cohorts, stratified by eGFR at baseline.
| eGFR <60 mL/min/1.73 m2 | eGFR ≥60 mL/min/1.73 m2 | |||||
|---|---|---|---|---|---|---|
| TIPS | LVP | P value | TIPS | LVP | P value | |
| (n = 93) | (n = 62) | (n = 45) | (n = 76) | |||
| Age (ys) | 59 (10) | 63 (12) | 0.01 | 55 (11) | 55 (11) | 0.9 |
| Female sex | 35 (38%) | 19 (31%) | 0.4 | 11 (24%) | 25 (33%) | 0.3 |
| White race | 79 (85%) | 47 (76%) | 0.2 | 38 (84%) | 59 (78%) | 0.4 |
| Non-Hispanic ethnicity | 87 (94%) | 56 (90%) | 0.5 | 43 (96%) | 71 (93%) | 0.6 |
| Co-morbidities* | ||||||
| Diabetes mellitus | 34 (37%) | 35 (56%) | 0.01 | 15 (33%) | 20 (26%) | 0.4 |
| Hypertension | 43 (46%) | 51 (82%) | <0.001 | 16 (36%) | 48 (63%) | 0.003 |
| Coronary artery disease | 28 (30%) | 16 (26%) | 0.6 | 7 (16%) | 11 (14%) | 0.9 |
| Etiology of cirrhosis | 0.4 | 0.02 | ||||
| NASH | 9 (10%) | 6 (10%) | 7 (16%) | 3 (4%) | ||
| Alcohol | 26 (28%) | 19 (31%) | 16 (36%) | 16 (21%) | ||
| HCV infection | 20 (22%) | 9 (15%) | 8 (18%) | 23 (30%) | ||
| Mixed etiology | 16 (17%) | 6 (10%) | 6 (13%) | 8 (11%) | ||
| Other | 22 (24%) | 22 (35%) | 8 (18%) | 26 (34%) | ||
| Listed for liver transplant | 37 (41%) | 27 (44%) | 0.8 | 16 (36%) | 23 (31%) | 0.5 |
| MELD score | 20 (8) | 20 (8) | 0.6 | 12 (5) | 13 (6) | 0.5 |
| No. paracenteses, per 90 d** | 6.3 (4.6) | 5.7 (3.8) | 0.5 | 4.9 (2.9) | 4.9 (3.6) | 0.9 |
| Prior history of encephalopathy*** | 40 (46%) | 26 (42%) | 0.6 | 16 (37%) | 43 (57%) | 0.04 |
| Mean arterial pressure (mmHg) | 79 (12) | 82 (15) | 0.3 | 78 (10) | 82 (15) | 0.07 |
| On furosemide**** | 66 (80%) | 39 (63%) | 0.03 | 36 (86%) | 58 (76%) | 0.2 |
| Furosemide dose***** (mg/d) | 75 (69) | 44 (64) | 0.01 | 60 (44) | 43 (41) | 0.04 |
| On spironolactone****** | 58 (71%) | 24 (39%) | <0.001 | 36 (86%) | 51 (67%) | 0.03 |
| Spironolactone dose (mg/d) | 91 (98) | 35 (63) | <0.001 | 129 (101) | 78 (77) | 0.01 |
| Laboratory data | ||||||
| Sodium (mmol/L) | 135 (5) | 134 (4) | 0.3 | 131 (5) | 133 (5) | 0.03 |
| SUN; mg/dL | 43 (22) | 43 (23) | 0.8 | 17 (8) | 16 (10) | 0.6 |
| Creatinine (mg/dL) | 2.1 (1.0) | 2.7 (1.9) | 0.03 | 0.9 (0.2) | 0.8 (0.2) | <0.001 |
| Baseline eGFR (mL/min/1.73 m2) | 36 (13) | 33 (16) | 0.1 | 85 (17) | 94 (18) | 0.01 |
| AST (U/L) | 105 (184) | 59 (88) | 0.04 | 85 (101) | 114 (95) | 0.1 |
| ALT (U/L) | 60 (104) | 36 (77) | 0.1 | 47 (66) | 76 (97) | 0.05 |
| Alkaline phosphatase (U/L) | 160 (127) | 147 (89) | 0.5 | 122 (60) | 257 (331) | <0.001 |
| Total bilirubin (mg/dL) | 3.9 (6.1) | 4.8 (7.9) | 0.5 | 2.3 (1.8) | 5.3 (6.2) | <0.001 |
| Albumin (g/dL) | 2.9 (0.6) | 2.9 (0.6) | 0.8 | 2.7 (0.5) | 2.8 (0.5) | 0.3 |
| International normalized ratio | 1.5 (0.5) | 1.5 (0.4) | 0.5 | 1.5 (0.3) | 1.5 (0.3) | 0.8 |
| White blood cells (103/µL) | 6.7 (3.9) | 7.1 (4.5) | 0.6 | 7.5 (3.3) | 7.5 (5.1) | 0.9 |
| Hemoglobin (g/dL) | 9.9 (1.5) | 9.9 (1.9) | 0.9 | 10.6 (1.7) | 10.7 (2.2) | 0.8 |
| Platelets (103/µL) | 107 (67) | 140 (104) | 0.03 | 132 (86) | 124 (88) | 0.7 |
eGFR (estimated glomerular filtration rate), TIPS (transjugular intrahepatic portosystemic shunt), LVP (large-volume paracentesis), NASH (non-alcoholic steatohepatitis), MELD (Model for End-Stage Liver Disease), AST (aspartate transferase), ALT (alanine transferase). SUN, serum urea nitrogen; HCV, hepatitis C virus
Note: Values for categorical variables are given as number (percentage); for continuous variables, as mean ± standard deviation. Baseline values were taken immediately before TIPS placement, or closest available to day 0 of follow-up for the LVP cohort. Conversion factors for units: bilirubin in mg/dL to µmol/L, ×17.1; creatinine in mg/dL to µmol/L, ×88.4; SUN in mg/dL to mmol/L, ×0.357;
Comorbidities were documented by International Classification of Diseases,-Ninth Revision, code prior to study enrollment per electronic database review.
Available for 107/138 subjects in the TIPS cohort and all subjects in the LVP cohort.
Available for 268/276 subjects.
Available for 263/276 subjects.
Converted to equivalent dose of furosemide if on another loop diuretic
Available for 262/276 subjects.
Trends in eGFR Over Time (Unadjusted Analysis)
Among TIPS recipients who were not dialysis dependent at the time of TIPS placement, 106 of 132 (80%) had an eGFR determined 90 days prior to TIPS placement and 80 of132 (61%) had an eGFR determined 90 days after TIPS placement. All 132 had an eGFR determined immediately pre- and post-TIPS. Among the non–dialysis-dependent subjects in the LVP cohort, 85 of 116 (73%) had an eGFR determined 90 days after the reference LVP date, and 69 of 116 (59%) had an eGFR determined 180 days after the reference LVP date.
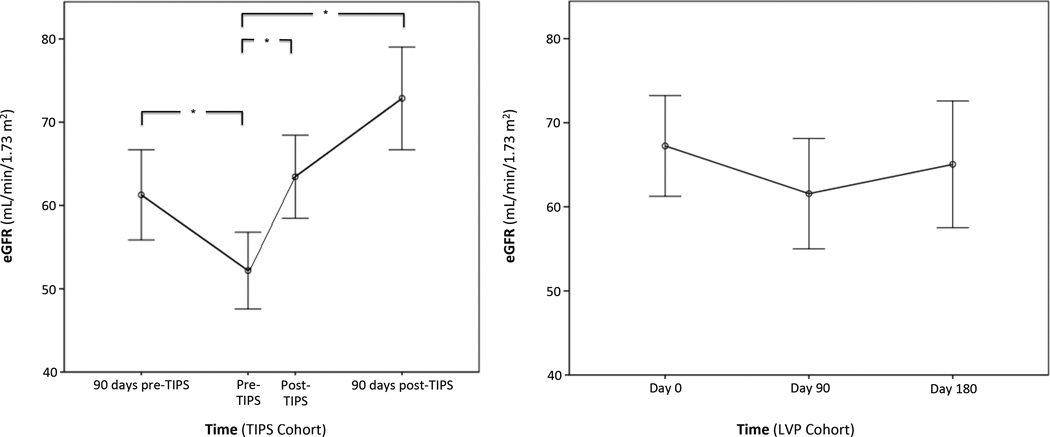
Unadjusted change in eGFR over time is presented in Figure 2 for the TIPS and LVP cohorts. For all subjects receiving TIPS, eGFR declined significantly from 90 days prior to TIPS to immediately pre-TIPS, decreasing from a mean of 63 ±28 to 53 ±27 mL/min/1.73 m2 (p < 0.001). The eGFR then improved immediately post-TIPS placement to 64 ±30 mL/min/1.73 m2 (p <0.001) and continued to increase to 75 ±28 mL/min/1.73 m2 90 days after TIPS placement (p <0.001). Serum creatinine followed the same statistical trend, in an inverse course.
Figure 2. Unadjusted change in glomerular filtration rate over time in TIPS (left) and LVP (right) cohorts.
Key: eGFR (estimated glomerular filtration rate), TIPS (transjugular intrahepatic portosystemic shunt), LVP (large-volume paracentesis).
* P <0.001.
P >0.4 for all comparisons in right panel
In contrast, for all subjects in the serial LVP cohort, eGFR did not change significantly from day 0 to day 90 of follow-up (72 ±33 to 66 ±31 mL/min/1.73 m2; p = 0.4) or in an additional follow-up period from day 90 to day 180 (66 ±31 to 68 ±31 mL/min/1.73 m2; p = 0.5). There was no significant change in serum creatinine during this period.
In a sensitivity analysis, 131 subjects who had eGFR determined at all time points (TIPS, n=66; serial LVP, n=65) were analyzed. Results were similar to those in the primary analysis. Another sensitivity analysis using a maximum-likelihood, mixed-effects repeated-measures model showed a significant change in eGFR over time (p <0.001), a significant interaction between TIPS placement and time (p <0.001), and no significant between-subject effect of TIPS placement (p = 0.2).
Change in eGFR After 90 Days (Adjusted Analysis)
In stratified multivariable linear regression, after adjusting for age, baseline eGFR, and etiology of cirrhosis, those with a baseline eGFR <60 mL/min/1.73 m2 had a significant improvement in eGFR after TIPS compared to treatment with serial LVP (21 [95% CI, 13–29] mL/min/1.73 m2; p <0.001). There was no significant difference in eGFR change after 90 days for those with baseline eGFR ≥60 mL/min/1.73 m2 (1 [95% CI, −9 to 12] mL/min/1.73 m2; p = 0.8; Table 3). There was significant effect modification between TIPS status and baseline eGFR (p = 0.001) across the entire cohort. There was no difference in these results in a sensitivity analysis of those listed for liver transplant or those not listed for liver transplant.
Table 3.
Multivariable model comparing TIPS vs. serial LVP to predict change in eGFR after 90 days, stratified by baseline eGFR and for all subjects
| Parameter Estimate (95% CI) |
P value | ||
|---|---|---|---|
| Baseline eGFR <60 mL/min/1.73 m2 | |||
| TIPS, vs serial LVP as reference | 21.27 (13.07 to 29.47) | <0.001 | |
| Age, per 1-y older | −0.21 | [−0.61, 0.20] | 0.3 |
| Baseline eGFR, per 1-mL/min/1.73 m2 greater | −0.08 | [−0.34, 0.19] | 0.6 |
| Etiology of cirrhosis, vs alcohol as reference | |||
| NASH | 6.25 | [−10.17, 22.66] | 0.5 |
| HCV infection | 11.37 | [−0.08, 22.66] | 0.05 |
| Mixed etiology | 9.34 | [−2.25, 20.92] | 0.1 |
| Other | 3.71 | [−6.87, 14.30] | 0.5 |
| Baseline eGFR ≥60 mL/min/1.73 m2 | |||
| TIPS, vs serial LVP as reference | 1.37 | [−9.48, 12.21] | 0.8 |
| Age, per 1-y older | −0.51 | [−1.09, 0.06] | 0.08 |
| Baseline eGFR, per 1-mL/min/1.73 m2 greater | −0.12 | [−0.45, 0.20] | 0.5 |
| Etiology of Cirrhosis, vs alcohol as reference | |||
| NASH | 3.38 | [−16.65, 23.41] | 0.7 |
| HCV infection | 3.66 | [−9.95, 17.27]] | 0.6 |
| Mixed etiology | 3.05 | [−13.48, 19.58] | 0.7 |
| Other | −3.68 | [−18.62, 11.28] | 0.6 |
| All Participants | |||
| TIPS, vs serial LVP as reference | 12.71 | [6.50, 18.92] | <0.001 |
| Baseline eGFR*, per 1-mL/min/1.73 m2 greater | 0.01 | [−0.13, 0.14] | 0.9 |
| Interaction Term: eGFR + TIPS | −0.33 | [−0.53, −0.13] | 0.001 |
| Age, per 1-y older | −0.32 | [−0.63, 0.00] | 0.05 |
| Etiology of cirrhosis, vs alcohol as reference | |||
| NASH | 2.83 | [−9.40, 15.06] | 0.7 |
| HCV infection | 6.96 | [−1.47, 15.39] | 0.1 |
| Mixed etiology | 6.13 | [−3.26, 15.52] | 0.2 |
| Other | 0.04 | [−8.22, 8.30] | 0.9 |
Baseline eGFR centered at 60 mL/min/1.73 m2 for interaction analysis.
Note: Baseline eGFR was measured immediately before TIPS placement, or closest available to day 0 of follow-up or the LVP cohort. Parameter Estimate refers to the expected change in eGFR (in mL/min/1.73 m2) over 90 days, with respect to each predictor, while holding other factors constant.
HCV, hepatitis C virus; TIPS (transjugular intrahepatic portosystemic shunt), LVP (large-volume paracentesis), eGFR (estimated glomerular filtration rate), CI (confidence interval), NASH (non-alcoholic steatohepatitis)
Death by 90 Days
Of all 276 subjects, 271 (98%) had a confirmed vital status at 90 days’ follow-up. Across all subjects with a confirmed vital status in the TIPS cohort, 40 of 133 (30%) died by 90 days after TIPS placement. Raw survival percentages for the entire cohort and stratified by baseline eGFR are presented in Table 4. In stratified multivariable logistic regression, after adjusting for age and eGFR, those treated with TIPS with a baseline eGFR <60 mL/min/1.73 m2 had a similar risk of death compared to treatment with serial LVP (odds ratio [OR], 1.30; 95% CI, 0.62–2.72; p = 0.5). Those treated with TIPS with a baseline eGFR ≥60 mL/min/1.73 m2 also had a similar risk of death compared to treatment with serial LVP (OR, 0.56; 95% CI, 0.22–1.40; p = 0.2; Table S1, available as online supplementary material). There was significant effect modification between TIPS status and baseline eGFR (p = 0.01) across the entire cohort. There was no difference in these results in a sensitivity analysis of those listed for liver transplant or those not listed for liver transplant.
Table 4.
Unadjusted mortality and adverse events during 90-day follow-up.
| Event | TIPS (n = 138) |
LVP (n = 138) |
P value |
|---|---|---|---|
| Death by 90 days | 40* (30%) | 46 (33%) | 0.6 |
| Need for renal replacement therapy** | 13 (10%) | 4 (3%) | 0.04 |
| Need for TIPS revision | 13 (10%) | -- | -- |
| Hepatic encephalopathy after TIPS | 48 (35%) | -- | -- |
| Contrast Nephropathy*** | 8 (6%) | -- | -- |
Note: Unless otherwise indicated, values are given as number (percentage)
TIPS (transjugular intrahepatic portosystemic shunt), LVP (large-volume paracentesis)
Excludes 6 subjects who were lost to follow-up at 90 days.
Excludes 6 subjects in the TIPS cohort and 19 subjects in the LVP cohort who were dialysis dependent at the start of the follow-up period.
Contrast nephropathy was determined by documentation by treating clinicians at the time of TIPS. All cases were seen in those with baseline eGFR <60 mL/min/1.73 m2.
Procedural Characteristics of TIPS Placement
All TIPS were placed endovascularly under fluoroscopic guidance by interventional radiologists. Mean portosystemic gradient decreased from 17 ±5 mmHg before TIPS to 7 ±3 mmHg after TIPS (p <0.001). Volume of intravenous contrast used was reported in 79 of 138 (57%) of TIPS procedures, and the mean was 138 ±80 mL. Sixty four percent of endoprostheses used were covered stents (Viatorr®, Gore Medical) and 36% were uncovered stents (Wallstent™, Boston Scientific). Ninety one percent of stents were 10 mm in diameter.
Other Outcomes at 90 Days
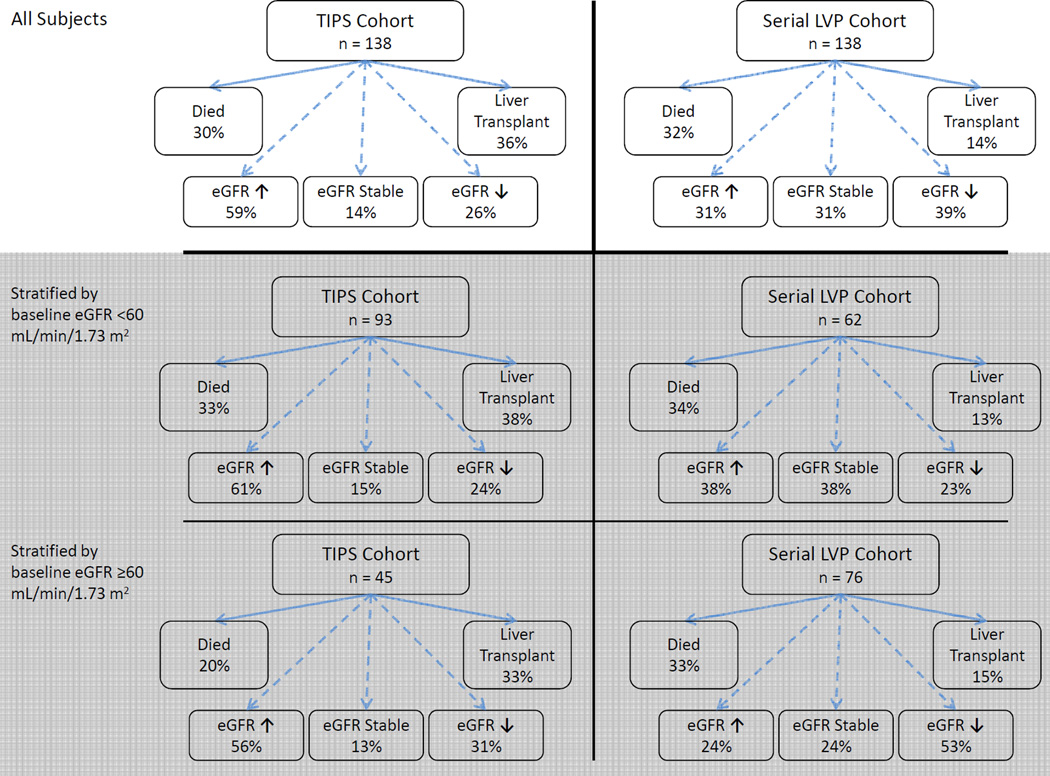
Among all subjects, 50 of 138 (36%) in the TIPS cohort and 19 of 138 (14%) in the LVP cohort went on to receive a liver transplant. After adjusting for age and eGFR, TIPS placement was associated with 3.51 (95% CI, 1.89–6.52) times higher odds of receiving a liver transplant compared to treatment with serial LVP (p <0.001). A conceptual diagram of raw 90-day outcomes, including death, rates of liver transplantation, and change in eGFR is presented in Figure 3. Among those with available data, in the TIPS cohort, mean follow-up MELD score at 90 days was 16 ±6 and mean total bilirubin was 4.0 ±5.7 mg/dL. In the LVP cohort, mean follow-up MELD score at 90 days was 16 ±9 and mean total bilirubin was 3.9 ±6 mg/dL. After adjusting for age, baseline eGFR, and etiology of cirrhosis, there was no difference between TIPS vs. LVP cohorts in change in serum sodium (−0.6 [95% CI, −2.2 to 1.1] mmol/L; p = 0.5), change in mean arterial pressure (−2 [95% CI, −7 to 3] mmHg; p = 0.4), change in furosemide dose (15 [95% CI, −3 to 32] mg/day; p = 0.1), or change in spironolactone dose (16 [95% CI, −10 to 42] mg/day; p = 0.2). Among 81 TIPS recipients with available data, the mean number of LVPs decreased from 5.5 ±3.9 in the 90 days before TIPS to 0.9 ±1.4 in the 90 days after TIPS (p <0.001). There was no significant correlation between change in eGFR and post-TIPS reduction of the portosystemic gradient (r = −0.10; p = 0.4) or degree of pre-TIPS portal hypertension (r = −0.19; p = 0.1).
Figure 3. Summary of raw outcomes at 90 days. eGFR was stable if the absolute change was ≤5 mL/min/1.73 m2.
Those requiring renal replacement during the follow-up period were included in the decreased eGFR group. Results are reported for all subjects (top panels), and stratified by baseline low eGFR (middle panels) or normal baseline eGFR (bottom panels). Key: eGFR (estimated glomerular filtration rate), TIPS (transjugular intrahepatic portosystemic shunt), LVP (large-volume paracentesis).
Multivariable models comparing TIPS vs serial LVP to predict death by 90 d, stratified by baseline eGFR and for all
Adverse Events Following TIPS Placement
Adverse events are reported in Table 4, including post-TIPS hepatic encephalopathy, need for renal replacement therapy, need for TIPS revision, and contrast nephropathy.
Discussion
Because of its potential to reverse the pathophysiologic changes of portal hypertension, TIPS has important treatment implications in patients with refractory ascites. To our knowledge, this is the largest study examining kidney function in TIPS recipients compared to treatment with serial LVP. We show that in multivariable analysis, TIPS placement for refractory ascites is associated with a greater improvement in eGFR compared to treatment with serial LVP, with significant effect modification between TIPS placement and baseline eGFR. This was highlighted in stratified analysis among those with a baseline eGFR <60 mL/min/1.73 m2.
We are aware of five randomized controlled studies that have previously compared TIPS placement to serial LVP in the treatment for refractory ascites.5–8, 13 These trials were designed to look at difference in mortality between the two treatments. They are small (ranging from 257 to 109 subjects8) and were all performed before the advent of covered TIPS stents, which may affect TIPS outcomes.41 Unfortunately, they have been not been consistent in their results: two showed a survival benefit with TIPS,5, 13 two show no difference in survival,6, 8 and one showed a survival benefit with serial LVP.7 Overall, meta-analyses of these trials have not shown a consistent survival benefit for either treatment.9–12, 42 However, a careful look at trends in eGFR may offer insight into which patients might benefit from TIPS, as kidney function is closely tied to mortality and is a key component of prognostic scores in cirrhosis.36, 43–47 Placement of TIPS has been associated with improvement in kidney function in small studies.17, 26, 27, 29, 30, 32, 48, 49 We were able to confirm and elaborate on this phenomenon using multivariable analysis. Analysis of eGFR before and after TIPS highlighted an interesting trend: eGFR declined significantly in the 90 days leading up to TIPS placement, followed by improvement in eGFR after TIPS. This clinical pattern highlights two important points. First, TIPS placement has the potential to reverse the decline in kidney function in this population. Second, there may be a practice preference among some hepatologists to refer patients for TIPS when a decline in eGFR limits treatment with serial LVP and diuretics. As such, diuretic doses were higher overall in the TIPS cohort, which may have also influenced eGFR in this group.
Overall, our study was not intended to advocate for or against TIPS placement for refractory ascites, but rather to make the important observation that TIPS has the potential to reverse the pathophysiology of portal hypertension that contributes to decreased kidney function. While this potential seems greatest for those with low baseline eGFR (for example, in those with hepatorenal syndrome), TIPS must be employed cautiously in this population, as these patients have high overall morbidity and may be prone to suffer adverse effects of TIPS placement. Only one prior non-randomized study has examined TIPS placement in those with low eGFR, and had promising results in 31 subjects.30 Our data supports further prospective study of TIPS in those with low eGFR.
While kidney function is important, we must consider overall mortality when assessing the role of TIPS for refractory ascites. In stratified analysis, there was a trend towards increased mortality in the TIPS cohort, and there was significant effect modification between TIPS status and baseline eGFR for this outcome. Even though the TIPS and LVP cohorts appeared statistically well matched, this was not a randomized trial, and thus subjects referred to TIPS may not have been drawn from the same population as those who were treated with serial LVP. It may be that relative improvement in those with lower baseline eGFR after TIPS may influence survival in this population. The two randomized trials that showed a survival benefit after TIPS showed a significant improvement in eGFR in the TIPS arm5 or a significant decline in eGFR in the LVP arm.13 The presence of effect modification between eGFR and TIPS placement further suggests that there is an important interaction involving baseline kidney function in the TIPS population that requires additional investigation.
Other outcomes after TIPS placement are important to consider, such as post-TIPS hepatic encephalopathy. Our post-TIPS encephalopathy rate of 36% is consistent with the existing literature.5–8, 13, 50 The TIPS patency rate was 90%, which is consistent with prior trials.1 Rates of liver transplantation were higher in those who received TIPS compared to those treated with serial LVP, which is consistent with a recent analysis of the UNOS liver transplant waitlist.51 Sensitivity analyses of liver transplant candidates in our study showed similar results to the entire cohort, suggesting that changes in eGFR were independent of transplant listing status.
There are advantages and disadvantages to the matching algorithm used in this study. Matching by MELD score provided a large, statistically similar group of subjects with ascites who were treated with serial LVP. In the absence of a randomization, prospective design, we believe this is an efficient and valid approximation for a retrospective study of this kind. However, as mentioned above, there may be clinical characteristics that are not fully captured by this statistical matching process, including practice patterns by treating clinicians, so it is likely not all subjects in the LVP cohort would have been considered for TIPS placement.
There are limitations that affect how this study should be interpreted. This is a retrospective study in which all significant findings should be viewed as associations that require prospective verification. Analysis was restricted to subjects with clinically available data, and as such, the sample size of the primary analysis was limited to those who had eGFR measured in follow-up. However, we were reassured that in those with data at all time points, results of the primary and secondary analyses were similar to the entire cohort, and a maximum-likelihood, mixed-effects repeated-measures model confirmed a significant interaction for change in eGFR with TIPS placement and time when analyzing all data points. Parameters such as urine sodium, urine protein, bladder pressure, and serum renin and aldosterone levels were not routinely available in this population, which would have helped to add insight into the causative mechanisms behind improved kidney function after TIPS. While there are limitations of creatininebased eGFR formulas in cirrhosis,52 multiple studies have shown that small changes in creatinine (using the AKIN threshold of ≥0.3 mg/dL) can predict survival, and are therefore meaningful.53 Stratified multivariable analysis, while providing clinically interpretable results, may have come at the expense of decreased power to detect significant outcomes, especially in models of death at 90 days. Overall, we believe this large cohort study furthers our understanding of change in eGFR after TIPS.
In conclusion, TIPS placement was associated with improvement in kidney function compared to treatment with serial LVP. This was most prominent in subjects with baseline eGFR <60 mL/min/1.73 m2. Prospective studies of TIPS in populations with eGFR <60 mL/min/1.73 m2 are needed to further evaluate these findings.
Supplementary Material
Acknowledgments
Dr Allegretti thanks E. Fran Cook, PhD, for his mentorship and assistance in framing the manuscript.
Support: Funding for this study was from departmental funds. No external sources influenced study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication. Dr Allegretti is supported by National Institutes of Health (NIH) grant 5T32DK007540-30; Dr Chung, by NIH grant K24 DK078772; and Dr Thadhani, by NIH grants R01 DK094486 and K24 DK094872.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: ASA, IB, ZI; data acquisition: ASA, GO; data analysis/interpretation: ASA, GO, JC; statistical analysis: ASA, JW; supervision or mentorship: RIT, RTC, ZI. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. ASA takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Supplementary Material
Table S1: Multivariable models comparing TIPS vs serial LVP to predict death by 90 days.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Supplementary Material Descriptive Text for Online Delivery
Supplementary Table S1 (PDF). Multivariable models comparing TIPS vs serial LVP to predict death by 90 days.
References
- 1.Rossle M, Gerbes AL. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: A critical update. Gut. 2010;59(7):988–1000. doi: 10.1136/gut.2009.193227. [DOI] [PubMed] [Google Scholar]
- 2.Gerbes AL. The patient with refractory ascites. Best Pract Res Clin Gastroenterol. 2007;21(3):551–560. doi: 10.1016/j.bpg.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006;55(Suppl 6):vi1–12. doi: 10.1136/gut.2006.099580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arroyo V, Gines P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. international ascites club. Hepatology. 1996;23(1):164–176. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 5.Rossle M, Ochs A, Gulberg V, et al. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med. 2000;342(23):1701–1707. doi: 10.1056/NEJM200006083422303. [DOI] [PubMed] [Google Scholar]
- 6.Gines P, Uriz J, Calahorra B, et al. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology. 2002;123(6):1839–1847. doi: 10.1053/gast.2002.37073. [DOI] [PubMed] [Google Scholar]
- 7.Lebrec D, Giuily N, Hadengue A, et al. Transjugular intrahepatic portosystemic shunts: Comparison with paracentesis in patients with cirrhosis and refractory ascites: A randomized trial. french group of clinicians and a group of biologists. J Hepatol. 1996;25(2):135–144. doi: 10.1016/s0168-8278(96)80065-1. [DOI] [PubMed] [Google Scholar]
- 8.Sanyal AJ, Genning C, Reddy KR, et al. The north american study for the treatment of refractory ascites. Gastroenterology. 2003;124(3):634–641. doi: 10.1053/gast.2003.50088. [DOI] [PubMed] [Google Scholar]
- 9.Albillos A, Banares R, Gonzalez M, Catalina MV, Molinero LM. A meta-analysis of transjugular intrahepatic portosystemic shunt versus paracentesis for refractory ascites. J Hepatol. 2005;43(6):990–996. doi: 10.1016/j.jhep.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 10.D'Amico G, Luca A, Morabito A, Miraglia R, D'Amico M. Uncovered transjugular intrahepatic portosystemic shunt for refractory ascites: A meta-analysis. Gastroenterology. 2005;129(4):1282–1293. doi: 10.1053/j.gastro.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 11.Saab S, Nieto JM, Ly D, Runyon BA. TIPS versus paracentesis for cirrhotic patients with refractory ascites. Cochrane Database Syst Rev. 2004;3(3):CD004889. doi: 10.1002/14651858.CD004889. [DOI] [PubMed] [Google Scholar]
- 12.Salerno F, Camma C, Enea M, Rossle M, Wong F. Transjugular intrahepatic portosystemic shunt for refractory ascites: A meta-analysis of individual patient data. Gastroenterology. 2007;133(3):825–834. doi: 10.1053/j.gastro.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Salerno F, Merli M, Riggio O, et al. Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology. 2004;40(3):629–635. doi: 10.1002/hep.20364. [DOI] [PubMed] [Google Scholar]
- 14.Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: Pathophysiology and management. Clin J Am Soc Nephrol. 2006;1(5):1066–1079. doi: 10.2215/CJN.01340406. [DOI] [PubMed] [Google Scholar]
- 15.Gines P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361(13):1279–1290. doi: 10.1056/NEJMra0809139. [DOI] [PubMed] [Google Scholar]
- 16.Gines P, Guevara M, Arroyo V, Rodes J. Hepatorenal syndrome. Lancet. 2003;362(9398):1819–1827. doi: 10.1016/S0140-6736(03)14903-3. [DOI] [PubMed] [Google Scholar]
- 17.Wong F, Sniderman K, Liu P, Blendis L. The mechanism of the initial natriuresis after transjugular intrahepatic portosystemic shunt. Gastroenterology. 1997;112(3):899–907. doi: 10.1053/gast.1997.v112.pm9041252. [DOI] [PubMed] [Google Scholar]
- 18.Quiroga J, Sangro B, Nunez M, et al. Transjugular intrahepatic portal-systemic shunt in the treatment of refractory ascites: Effect on clinical, renal, humoral, and hemodynamic parameters. Hepatology. 1995;21(4):986–994. [PubMed] [Google Scholar]
- 19.Wong F, Sniderman K, Liu P, Allidina Y, Sherman M, Blendis L. Transjugular intrahepatic portosystemic stent shunt: Effects on hemodynamics and sodium homeostasis in cirrhosis and refractory ascites. Ann Intern Med. 1995;122(11):816–822. doi: 10.7326/0003-4819-122-11-199506010-00002. [DOI] [PubMed] [Google Scholar]
- 20.Wong W, Liu P, Blendis L, Wong F. Long-term renal sodium handling in patients with cirrhosis treated with transjugular intrahepatic portosystemic shunts for refractory ascites. Am J Med. 1999;106(3):315–322. [PubMed] [Google Scholar]
- 21.Gerbes AL, Gulberg V, Waggershauser T, Holl J, Reiser M. Renal effects of transjugular intrahepatic portosystemic shunt in cirrhosis: Comparison of patients with ascites, with refractory ascites, or without ascites. Hepatology. 1998;28(3):683–688. doi: 10.1002/hep.510280313. [DOI] [PubMed] [Google Scholar]
- 22.Gines A, Escorsell A, Gines P, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105(1):229–236. doi: 10.1016/0016-5085(93)90031-7. [DOI] [PubMed] [Google Scholar]
- 23.Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56(9):1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the international club of ascites. Gut. 2015;64(4):531–537. doi: 10.1136/gutjnl-2014-308874. [DOI] [PubMed] [Google Scholar]
- 25.Somberg KA, Lake JR, Tomlanovich SJ, LaBerge JM, Feldstein V, Bass NM. Transjugular intrahepatic portosystemic shunts for refractory ascites: Assessment of clinical and hormonal response and renal function. Hepatology. 1995;21(3):709–716. [PubMed] [Google Scholar]
- 26.Martinet JP, Fenyves D, Legault L, et al. Treatment of refractory ascites using transjugular intrahepatic portosystemic shunt (TIPS): A caution. Dig Dis Sci. 1997;42(1):161–166. doi: 10.1023/a:1018861827399. [DOI] [PubMed] [Google Scholar]
- 27.Testino G, Ferro C, Sumberaz A, et al. Type-2 hepatorenal syndrome and refractory ascites: Role of transjugular intrahepatic portosystemic stent-shunt in eighteen patients with advanced cirrhosis awaiting orthotopic liver transplantation. Hepatogastroenterology. 2003;50(54):1753–1755. [PubMed] [Google Scholar]
- 28.Schroeder ET, Anderson GH, Jr, Smulyan H. Effects of a portacaval or peritoneovenous shunt on renin in the hepatorenal syndrome. Kidney Int. 1979;15(1):54–61. doi: 10.1038/ki.1979.8. [DOI] [PubMed] [Google Scholar]
- 29.Brensing KA, Textor J, Strunk H, Klehr HU, Schild H, Sauerbruch T. Transjugular intrahepatic portosystemic stent-shunt for hepatorenal syndrome. Lancet. 1997;349(9053):697–698. doi: 10.1016/s0140-6736(97)24010-9. [DOI] [PubMed] [Google Scholar]
- 30.Brensing KA, Textor J, Perz J, et al. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: A phase II study. Gut. 2000;47(2):288–295. doi: 10.1136/gut.47.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guevara M, Gines P, Bandi JC, et al. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: Effects on renal function and vasoactive systems. Hepatology. 1998;28(2):416–422. doi: 10.1002/hep.510280219. [DOI] [PubMed] [Google Scholar]
- 32.Jalan R, Redhead DN, Thomas HW, et al. Mechanisms of changes in renal handling of sodium following transjugular intrahepatic portal systemic stent-shunt (TIPSS) Eur J Gastroenterol Hepatol. 1996;8(11):1111–1116. doi: 10.1097/00042737-199611000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Stanley AJ, Redhead DN, Bouchier IA, Hayes PC. Acute effects of transjugular intrahepatic portosystemic stent-shunt (TIPSS) procedure on renal blood flow and cardiopulmonary hemodynamics in cirrhosis. Am J Gastroenterol. 1998;93(12):2463–2468. doi: 10.1111/j.1572-0241.1998.00705.x. [DOI] [PubMed] [Google Scholar]
- 34.Huonker M, Schumacher YO, Ochs A, Sorichter S, Keul J, Rossle M. Cardiac function and haemodynamics in alcoholic cirrhosis and effects of the transjugular intrahepatic portosystemic stent shunt. Gut. 1999;44(5):743–748. doi: 10.1136/gut.44.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lotterer E, Wengert A, Fleig WE. Transjugular intrahepatic portosystemic shunt: Short-term and long-term effects on hepatic and systemic hemodynamics in patients with cirrhosis. Hepatology. 1999;29(3):632–639. doi: 10.1002/hep.510290302. [DOI] [PubMed] [Google Scholar]
- 36.Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 37.McMahon GM, Zeng X, Waikar SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821–1828. doi: 10.1001/jamainternmed.2013.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rhee CM, Bhan I, Alexander EK, Brunelli SM. Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism. Arch Intern Med. 2012;172(2):153–159. doi: 10.1001/archinternmed.2011.677. [DOI] [PubMed] [Google Scholar]
- 39.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Anonymous] [Google Scholar]
- 41.Perarnau JM, Le Gouge A, Nicolas C, et al. Covered vs. uncovered stents for transjugular intrahepatic portosystemic shunt: A randomized controlled trial. J Hepatol. 2014;60(5):962–968. doi: 10.1016/j.jhep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 42.European Association for the Study of the Liver: EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53(3):397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Lee M, Lee JH, Oh S, et al. CLIF-SOFA scoring system accurately predicts short-term mortality in acutely decompensated patients with alcoholic cirrhosis: A retrospective analysis. Liver Int. 2015;35(1):46–57. doi: 10.1111/liv.12683. [DOI] [PubMed] [Google Scholar]
- 44.Cholongitas E, Senzolo M, Patch D, Shaw S, O'Beirne J, Burroughs AK. Cirrhotics admitted to intensive care unit: The impact of acute renal failure on mortality. Eur J Gastroenterol Hepatol. 2009;21(7):744–750. doi: 10.1097/MEG.0b013e328308bb9c. [DOI] [PubMed] [Google Scholar]
- 45.Tsien CD, Rabie R, Wong F. Acute kidney injury in decompensated cirrhosis. Gut. 2013;62(1):131–137. doi: 10.1136/gutjnl-2011-301255. [DOI] [PubMed] [Google Scholar]
- 46.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426–1437. 1437.e1–1437.e9. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 47.Alessandria C, Ozdogan O, Guevara M, et al. MELD score and clinical type predict prognosis in hepatorenal syndrome: Relevance to liver transplantation. Hepatology. 2005;41(6):1282–1289. doi: 10.1002/hep.20687. [DOI] [PubMed] [Google Scholar]
- 48.Schroeder ET, Anderson GH, Jr, Smulyan H. Effects of a portacaval or peritoneovenous shunt on renin in the hepatorenal syndrome. Kidney Int. 1979;15(1):54–61. doi: 10.1038/ki.1979.8. [DOI] [PubMed] [Google Scholar]
- 49.Michl P, Gulberg V, Bilzer M, Waggershauser T, Reiser M, Gerbes AL. Transjugular intrahepatic portosystemic shunt for cirrhosis and ascites: Effects in patients with organic or functional renal failure. Scand J Gastroenterol. 2000;35(6):654–658. doi: 10.1080/003655200750023642. [DOI] [PubMed] [Google Scholar]
- 50.Rossle M, Gerbes AL. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: A critical update. Gut. 2010;59(7):988–1000. doi: 10.1136/gut.2009.193227. [DOI] [PubMed] [Google Scholar]
- 51.Berry K, Lerrigo R, Liou IW, Ioannou GN. Association between transjugular intrahepatic portosystemic shunt and survival in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 52.Belcher JM, Parikh CR, Garcia-Tsao G. Acute kidney injury in patients with cirrhosis: Perils and promise. Clin Gastroenterol Hepatol. 2013;11(12):1550–1558. doi: 10.1016/j.cgh.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egerod Israelsen M, Gluud LL, Krag A. Acute kidney injury and hepatorenal syndrome in cirrhosis. J Gastroenterol Hepatol. 2015;30(2):236–243. doi: 10.1111/jgh.12709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.