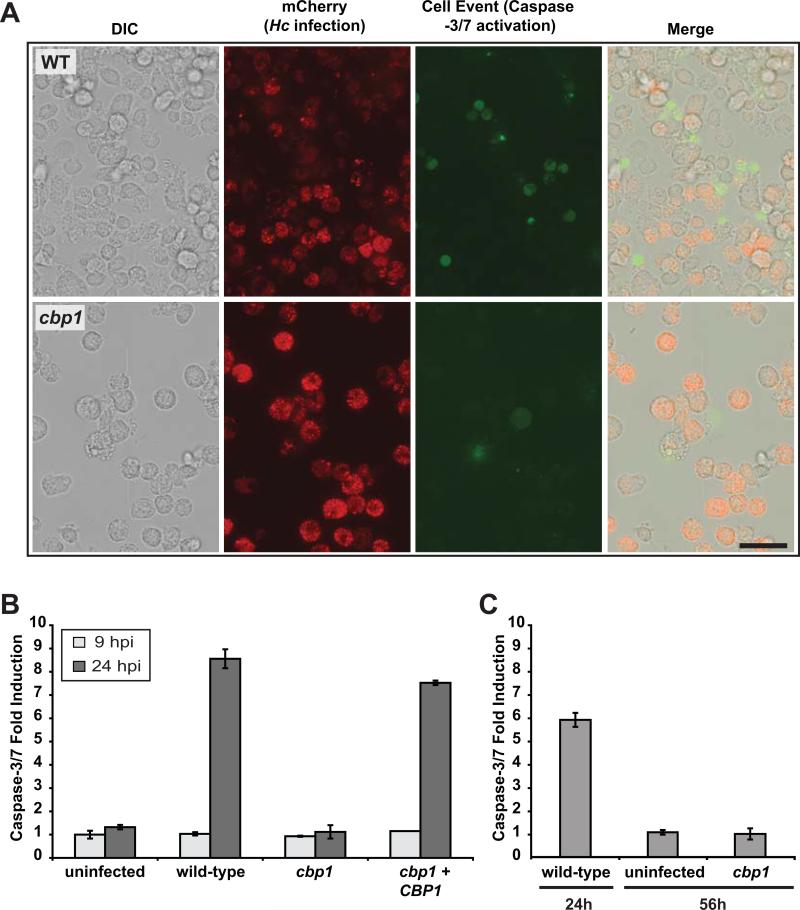
Figure 8. Cbp1 is required to promote caspase-3/7 activation in BMDMs infected with H. capsulatum yeast cells.
(A) Wild-type G217B ura5Δ or cbp1 mutant cells, each transformed with a construct expressing secreted mCherry, were used to infect BMDMs. Two days post-infection, infected BMDMs were incubated with CellEvent caspase-3/7 detection reagent. Scale bar corresponds to 50 μm. (B) BMDMs were either mock-infected (uninfected) or infected with either wild-type G217B ura5Δ+URA (wild-type), cbp1 mutant +URA (cbp1), or CBP1 complemented (cbp1+CBP1) yeast cells. At 9 and 24 hpi, BMDM lysates (containing 100 μg of total protein) were combined with DEVD-pNA colorimetric substrate, and cleavage of the substrate by active caspase-3/7 was monitored. Fold induction relative to mock-infected BMDMs at 9 hpi is shown and is representative of two independent experiments. (C) BMDMs were either mock-infected (uninfected) or infected with wild-type or cbp1 mutant cells. At the indicated time post-infection, BMDM lysates (containing 100 μg of total protein) were combined with DEVD-pNA colorimetric substrate, and cleavage of the substrate by active caspase-3/7 was monitored. Fold infection relative to mock-infected at 24 hpi is graphed. Although infection with wild-type cells resulted in a strong signal by 24 hpi followed by host-cell lysis, cbp1-infected cells did not show appreciable caspase-3/7 activation even at 56hpi.