Abstract
Hepatorenal syndrome (HRS) plays an important role in patients with liver cirrhosis on the wait list for liver transplantation (LT). The 1 and 5-year probability of developing HRS in cirrhotic with ascites is 20% and 40%, respectively. In this article, we reviewed current concepts in HRS pathophysiology, guidelines for HRS diagnosis, effective treatment options presently available, and controversies surrounding liver alone vs simultaneous liver kidney transplant (SLKT) in transplant candidates. Many treatment options including albumin, vasoconstrictors, renal replacement therapy, and eventual LT have remained a mainstay in the treatment of HRS. Unfortunately, even after aggressive measures such as terlipressin use, the rate of recovery is less than 50% of patients. Moreover, current SLKT guidelines include: (1) estimation of glomerular filtration rate of 30 mL/min or less for 4-8 wk; (2) proteinuria > 2 g/d; or (3) biopsy proven interstitial fibrosis or glomerulosclerosis. Even with these updated criteria there is a lack of consistency regarding long-term benefits for SLKT vs LT alone. Finally, in regards to kidney dysfunction in the post-transplant setting, an estimation of glomerular filtration rate < 60 mL/min per 1.73 m2 may be associated with an increased risk of patients having long-term end stage renal disease. HRS is common in patients with cirrhosis and those on liver transplant waitlist. Prompt identification and therapy initiation in transplant candidates with HRS may improve post-transplantation outcomes. Future studies identifying optimal vasoconstrictor regimens, alternative therapies, and factors predictive of response to therapy are needed. The appropriate use of SLKT in patients with HRS remains controversial and requires further evidence by the transplant community.
Keywords: Liver transplantation, Simultaneous liver kidney transplantation, Vasopressors, Dialysis, Post-transplant outcomes, Hepatorenal syndrome
Core tip: We aim to review the literature on hepatorenal syndrome (HRS) in the setting of liver transplantation (LT) and address critical issues that are barriers to improved outcomes. Many consistencies have remained as treatment options including albumin, vasoconstrictors, renal replacement therapy, and eventual LT. Moreover, the utility of simultaneous liver kidney transplantation in HRS patients still requires further evidence by the transplant community.
INTRODUCTION
Prior to diagnosing a patient with hepatorenal syndrome (HRS) in the setting of liver transplantation (LT), it is important to rule out other etiologies of renal dysfunction. A broad differential should include reversible causes such as acute kidney injury (AKI) or acute tubular necrosis (ATN) and irreversible cause like chronic kidney disease (CKD) or parenchymal kidney disease.
Traditionally there are three types of AKI (pre-renal azotemia, intrinsic kidney disease, and post-obstructive causes) that are still common in patients with liver disease in addition to HRS[1]. Common causes of pre-renal injury independent of HRS include infection, intravascular fluid depletion, GI fluid losses, surgery or bleeding, and renal artery occlusion[2], all of which should appropriately respond to volume expansion with albumin within 48 h. If there is any recent contrast media or nephrotoxic agent with granular casts and proteinuria > 500 mg, it is important to consider ATN as a likely diagnosis[3]. A recent study evaluating patients with AKI [pre-renal azotemia (n = 35), HRS (n = 35), ATN (n = 36)] revealed that pre-renal azotemia has a lower mortality when compared to both HRS (P = 0.05) and ATN (P = 0.04)[4].
Intrinsic kidney disease is more common than previously believed in the cirrhotic population, and is thought to be related to the underlying etiology of cirrhosis[2]. Kidney biopsy is most useful in intrinsic kidney disease with hematuria (50 red blood cells per high power field), proteinuria (> 500 mg/d), renal insufficiency of unknown origin, or HRS for a prolonged period of time. Histologically, IgA nephropathy, membrano-proliferative glomerulonephritis, focal global glomerulosclerosis, and diabetic nephropathy[5] are the most common biopsy findings. The importance of diagnosing parenchymal disease is especially important if a patient is being considered for combined liver-kidney transplantation[6]. Additionally, obstructive causes of renal dysfunction including nephrolithiasis, bladder outlet obstruction and other intra-abdominal etiologies should be assessed.
If the aforementioned workup returns negative, HRS should be considered as a potential cause of renal dysfunction. The 1-year and 5-year probability of developing HRS in patients with ascites is 20% and 40%, respectively[7]. The most recent diagnostic criteria for HRS from the International Ascites Club (IAC) in 2007 include creatinine (Cr) > 1.5 mg/dL, no improvement of Cr after volume expansion with albumin after 48 h, no current or recent exposure to nephrotoxic drugs, absence of parenchymal disease (proteinuria > 500 mg/d), microscopic hematuria (50 red blood cells per high power field), and abnormal renal ultrasonography[8].
In this review, we will focus on various aspects of HRS and its impact on various phases of LT. Literature was searched for this review from various search engines including PubMed, Cochrane, and Scopus. Each of the citations for the papers originally pulled was then reviewed for additional articles for inclusion.
ROLE OF CR AND OTHER MARKERS OF RENAL IMPAIRMENT IN CIRRHOTICS
There is concern that serum Cr may not reflect accurate kidney function in the setting of HRS with significant liver dysfunction[8,9]. Cr is an indirect measure of renal function as it is derived from non-enzymatic conversion of creatine, which is stored in muscle and being produced within the liver. As patients develop cirrhosis there is increased muscle wasting, decreased protein intake, and diminished creatine synthesis resulting in overestimation of renal function[9,10]. Moreover, two individuals with similar glomerular filtration rates may have varying Cr levels due to variation associated with age, sex, race, body mass index, and bilirubin concentrations[11]. For example, women generally have lower serum Cr levels compared to men resulting in lower median MELD scores (14 vs 15, P < 0.001) and a higher likelihood to die on the transplant list when compared to the pre-MELD era[12].
Multiple mathematical formulas have been developed to utilize serum Cr to calculate an estimation of glomerular filtration rate (eGFR). These include Cockcroft-Gault (C-G) and Modification of Diet in Renal Disease (MDRD) which incorporate different variables. C-G requires age, gender, weight, and serum Cr, while MDRD-4 utilizes age, gender, ethnicity, and serum Cr and MDRD-6 also involves albumin and urea[13,14]. In our cirrhotic population, MDRD-6 is used more widely when compared to C-G given inclusion of albumin and urea. Moreover, exogenous markers such as inulin have been previously documented to improve accuracy when determining renal function. Unfortunately the “gold standard” inulin infusion technique is time consuming, expensive, and potentially invasive making it a less viable option[15].
Multiple AKI biomarkers including NGAL, Cystatin C, IL-18, NAG, and KIM-1 have been well characterized and may delineate patients who have the risk of progression of disease and will require renal replacement therapy (RRT)[2,16,17]. For example, Aberg et al[18] looked specifically at the urinary marker neutrophil gelatinase-associated lipocalin (NGAL) in 203 LT patients and demonstrated that raised urinary levels of NGAL independently predicted pre-LT kidney dysfunction in the setting of HRS and could have the potential to help decide the need to performed combined liver-kidney transplantation. Additionally, urinary NGAL levels to be a strong predictor for short-term mortality, with HRS patients having intermediate levels between prerenal azotemia and intrinsic AKI[19]. Furthermore, certain studies have also shown cystatin C level may be an important marker for predicting mortality in HRS[20,21]. However, it is important to note that at this time current IAC or Acute Dialysis Quality Initiative do not recommend evaluating for these biomarkers.
BRIEF PATHOPHYSIOLOGY AND TYPES OF HRS
The pathophysiology of HRS has been well documented previously with portal hypertension leading to splanchnic artery dilatation[22,23]. This phenomenon results in a number of downstream effects including arterial underfilling, increased cardiac output, and vasoconstriction of renal arteries[8]. Ultimately the kidneys respond with increased activity of renin-angiotensin-aldosterone system as well as non-osmotic release of vasopressin, both of which result in worsening GFR, ascites, and hemodynamic instability[24,25].
HRS is typically divided into two subtypes, type 1 and type 2, based on the rate of progression of renal disease and prognosis. Diagnostic criteria for type 1 HRS (in addition to criteria for HRS according to IAC mentioned above) include serum Cr > 2.5 mg/dL, doubling of serum Cr in less than 2 wk, no history of diuretic resistant ascites, and generally a precipitating event. On the other hand, type 2 HRS is a gradually progressive renal impairment without any precipitating events and usually associated with diuretic resistant ascites. Additionally, patient outcomes in terms of survival were reported to be better with type 2 HRS vs type 1[26].
CLASSIFICATIONS OF RENAL DYSFUNCTION IN PATIENTS WITH CIRRHOSIS
Various criteria are used for classification of renal dysfunction in patients with liver cirrhosis. Two of the most commonly used criteria include the Risk, Injury, Failure, Loss, and End-Stage Kidney Disease (RIFLE) and AKI network (AKIN). The RIFLE criteria utilize both serum Cr level and urine output to assess what stage of renal injury has occurred. For example, acute renal injury is Cr doubled from baseline and urine output < 0.5 mL/kg per hour over 12 h while acute renal failure is Cr tripled from baseline and urine output < 0.3 mL/kg per hour over 24 h. A major limitation of the RIFLE classification is that per these criteria a large number of cirrhotic patients would already present with some degree of AKI. In 2007 the AKIN has proposed a new definition of AKI that condenses RIFLE into 3 stages to increase sensitivity and specificity of diagnosing AKI. Moreover, the Kidney Disease Improving Global Outcomes recently defined AKI as diminished kidney function resulting in 0.3 mg/dL increase in serum Cr in 48 h, or a 50% increase in baseline Cr (within 7 d), or a urine volume of < 0.5 mL/kg per hour for 6 h[8,27]. It has been well documented that approximately 20% of patients hospitalized for decompensated cirrhosis present with a concomitant AKI[28]. This phenomenon is related to the progressive vasodilatory state of cirrhosis causing a decrease in arterial volume and resultant vasoconstriction of renal vessels. Interestingly, two prospective studies assessing AKI criteria in patients with cirrhosis found that AKI with serum Cr values < 1.5 mg/dL is a relatively benign and potentially reversible condition, while significant increase in Cr (> 1.5 mg/dL) is associated with a worse prognosis[29,30].
A retrospective study utilized the RIFLE classification to look at 283 patients who underwent LT and stratified them into three cohorts: Risk, injury, and failure. Moreover, the failure group was further subdivided by etiology (HRS vs ATN) and the clinical course was followed for 5 years. Comparing these groups, the ATN group had significantly worse 1- and 5-year survival and renal outcomes, with an increased incidence of stage 4 and 5 CKD[31]. While only a single-center retrospective study, it is instrumental in demonstrating that the etiology of AKI may be more important than initially thought in predicting renal recovery[32].
Prerenal injury, ATN, and HRS encompass close to 80% of AKI etiology in the in the pre-transplantation setting[33]. A United Network for Organ Sharing (UNOS) based study in 2002 found that 40% of LT candidates have kidney dysfunction, best defined as a GFR < 60 cm3/min per square meter[34]. More recently, a prospective study following 463 patients classified renal failure into four main categories: Infections (n = 213, 46%), hypovolemia associated renal failure (n = 149, 32%), HRS (n = 60, 13%), parenchymal nephropathy (n = 41, 9%)[35]. While this is a simple classification, it is useful to assess prognosis and decisions regarding LT.
PREVALENCE AND PRECIPITANTS OF HRS IN WAIT-LIST AND TRANSPLANT PATIENTS
The prevalence of HRS has been reported to increase with severity and duration of cirrhosis. Ginès et al[36] studied 229 patients with cirrhosis and found an 18% incidence of HRS at one year, with an increase to 39% within five years. Additionally, Wong et al[37] reported HRS in 48% of patients on the LT waiting list, indicating an increased prevalence with disease progression. Various precipitants of HRS include spontaneous bacterial peritonitis, large volume paracentesis with inadequate albumin replacement, use of nephrotoxic drugs and hypovolemia due to bleeding and or dehydration. With the help of early diagnosis and aggressive management with vasopressors the incidence of HRS may decrease with an improvement in overall outcomes[38].
MANAGEMENT OF HRS
Medical management of HRS has been shown to improve short-term outcomes; however, long term outcomes are dismal without LT. Current medical treatment includes avoidance of HRS precipitants and pharmacological management prior to considering transjugular intrahepatic portosystemic shunt (TIPS) and RRT. Pharmacological treatment serves as a bridge to transplantation to improve the patient’s prognosis. There is a consensus on general measures in treating HRS including suspension of diuretic therapy, avoidance of nephrotoxic drugs and adjustment in doses of drugs. Moreover, per AASLD guidelines the role of albumin after large volume paracentesis (8 g of albumin for each liter of ascites removed) has been the standard of care.
Role of terlipressin in HRS
Given the significance of arterial vasodilatation in the pathophysiology of HRS, vasoconstrictors along with albumin have improved renal function in approximately 40%-60% of patients with type 1 HRS (Table 1)[39]. Terlipressin plus albumin has been shown to improve renal function in 35%-40% of patients with type 1 HRS, with initial IV boluses of 0.5-1 mg every 4 h that can be titrated to 3 mg every 4 h if there is limited response[40-42]. A study comparing terlipressin bolus vs continuous infusion found that while the rate of response was not statistically significant, the rate of adverse of events was lower in the infusion group with lower associated dosing[43].
Table 1.
The role of terlipressin and albumin in hepatorenal syndrome-1
| Ref. | Terlipressin dose | Albumin | Length | Terlipressin group: Cr (mg/dL) or Cr Cl (mL/min) | Control group: Cr (mg/dL) or Cr Cl (mL/min) | 30 d survival (terlipressin vs control) | Transplant free outcome |
| Hadengue et al[113] | 1 mg twice daily | No | 2 d | Cr Cl: 27 ± 4 | Cr Cl: 15 ± 2 | N/A | N/A |
| Halimi et al[49] | 4 mg/d | Yes | 7 d (mean) | Decline in Cr from 31%-75% from day 0 to day 5 | N/A | 13/18 (72%) patient response | N/A |
| Danalioglu et al[42] | 2-4 mg/d | Yes | 6 d | N/A | N/A | 20% vs 0% | N/A |
| Testro et al[54] | 1 mg every 6 h (max of 8 mg/d) | Yes | 12 d | N/A | N/A | 17/49 HRS type 1, 4/20 HRS type 2 | All transplant free outcomes responded to terlipressin |
| Sanyal et al[46] | 1 mg every 6 h (doubled on 4 d if Cr did not < 30%) | No (control group received albumin) | 14 d | Cr < 1.5 mg/dL (19/59, 33.9%) | Cr < 1.5 mg/dL (7/56, 12.5%) | N/A | 42.9% (24/56) vs 37.5% (21/56) in terlipressin vs control group at 180 d |
| von Kalckreuth et al[47] | 3.9 mg ± 1.3 mg (responders) vs 3.4 mg ± 1.4 mg (nonresponders) | Yes | 6 ± 4.9 d (responder) vs 8 ± 6.3 d (non-responders) | N/A | N/A | Complete response by day 7 was 52%, while at day 17 it was 84% | 25/38 (66%) of treatment complete response was achieved |
| Boyer et al[44] | 1 mg every 6 h | Yes | 6.3 d (mean) | Cr: 2.8 mg/dL | Cr: 3.8 mg/dL | N/A | 34% non-transplanted survival 100% transplant survival at 180 d |
| Hinz et al[51] | 2-6 mg/d | Yes | N/A | N/A | N/A | 57% of patients (12/21) responded to terlipressin. Age was a negative predictor for treatment response | No difference seen in mortality between responders and non-responders at 60 d |
| Heidemann et al[50] | 26.43 ± 30.86 (total dose for responders) vs 32.11 ± 31.57 (total dose for non-responders) | Yes | 9 d (responders) vs 10.5 d (non-responders) | N/A | N/A | One month survival was longer in responders vs non-responders (P = 0.048) | N/A |
| Sagi et al[45] (meta-analysis) | N/A | Yes | Minimum of 3 d of terlipressin | Cr must have been < 1.5 mg/dL at treatment end | N/A | Four trials (n = 223) with RR for reversal in type 1 HRS with terlipressin was 3.66 (95%CI: 2.15-6.23) | N/A |
| Fabrizi et al[48] (meta-analysis) | N/A | N/A | N/A | N/A | N/A | Five trials (n = 243 patients) with pooled OR of HRS reversal was 8.09 (95%CI: 3.52; 18.59) | Recovery of renal function occurs in less than 50% of patients with HRS even with terlipressin |
Cr: Creatinine; Cr Cl: Creatinine clearance; HRS: Hepatorenal syndrome; N/A: Not available.
While many studies demonstrate the use of terlipressin as a bridge to transplantation, it is important to note that fewer than 50% of patients who used terlipressin in the setting of HRS recover from a renal standpoint. One study assessed the efficacy of terlipressin plus albumin vs albumin alone for treatment of HRS-1 in the setting of LT. The 6-mo survival rate for those in the terlipressin group was 100% for transplanted patients and 34% for non-transplanted patients, while in the control group survival was 94% for transplanted patients and 17% for non-transplanted patients[44]. This study was able to show that terlipressin likely improved pre-transplant renal function while having no significant impact on post-transplant survival. On the other hand, Sagi et al[45] concluded improved transplant-free survival at 90 d (RR = 1.86, 95%CI: 1.0-3.4, P = 0.05) in those in the terlipressin arm when studying 223 patients in 4 separate trials. A prospective, randomized, double-blind, placebo-controlled clinical trial showed that terlipressin group showed Cr improvement from baseline to day 14 while on the treatment[46]. It appears that terlipressin treatment beyond one week and up to 20 d has the potential for further improvement[47]. Moreover, a recent meta-analysis of randomized trials (5 trials, n = 243 patients), showed the overall rate of patients on terlipressin with HRS who recovered renal function was 8.09 (95%CI: 3.52-18.59, P < 0.001[48]).
One study found a better response to terlipressin in the setting of higher serum sodium concentrations and lower serum bilirubin at the beginning of treatment[49], which would indicate that the early identification and treatment of HRS-1 may improve outcomes. A larger study was able to identify independent predictors of survival in the setting of terlipressin including age, duration of treatment, MELD score, and alcoholic cirrhosis[50], while an additional study was able to identify low urinary sodium prior to treatment being associated with poor survival[51].
Similar to the type 1 HRS patient population, terlipressin has been shown to improve renal function in type 2 HRS (Cr improvement in 8 out of 11 patients) when compared to organic renal disease[52]. Interestingly, a recent study examined 56 patients awaiting LT who were diagnosed with type 2 HRS. A subset of patients were being treated with terlipressin and albumin, but no differences were found in mortality in peri-operative setting or in post-transplantation outcomes (AKI, need for RRT, or development of CKD) when compared to the control group[53]. Moreover, another study also showed no benefit in using terlipressin in the setting of type 2 HRS[54]. Furthermore, while LT helps reverse type 2 HRS, there may be an association with longer intensive care stays and early-post-transplant CKD stage 3[55].
Role of other vasoconstrictors in HRS
Terlipressin is not available in United States; therefore midodrine, octreotide, ornipressin and noradrenaline with albumin have been used in uncontrolled studies to treat HRS. It was found that HRS patients were more likely to improve while treated with AVP when compared to octreotide alone[56]. Another study assessed the effect of octreotide, midodrine, and albumin on survival compared to control populations and found improved renal function and short-term survival in the setting of both HRS-1 and HRS-2[57]. With use of a combo of octreotide, midodrine and albumin, reversal of HRS has been reported to be as high as 40%[58].
Ornipressin is another potent splanchnic vasoconstrictor, but has been shown to have a higher incidence of vascular complications when compared to terlipressin[59]. In regards to noradrenaline, an unblinded study in 2007 was able to show that noradrenaline is an effective alternative to terlipressin in the setting of HRS type 1[60]. A more recent meta-analysis looked at 4 smaller studies where 154 patients were included and found that there was no difference between noradrenaline and terlipressin in regards to mortality at 30 d (RR = 0.89, 95%CI: 0.68 to 1.17) and reversal of HRS (RR = 0.97, 95%CI: 0.76 to 1.23)[61].
A recently published randomized study directly compared terlipressin with albumin to midodrine plus octreotide with albumin[62]. Terlipressin group was found to be significantly more effective in improving kidney function in HRS patients (70% vs 28%)[62]. Additionally, a small study that looked at three patients who were initially on terlipressin and attempted to switch treatment to midodrine plus octreotide on multiple attempts were found to have serum Cr elevation as well as diminished urine output[63].
Other treatment options for HRS
Among other options available for HRS management, TIPS has been increasingly utilized. While it is well documented that TIPS is effective treatment for refractory variceal bleeding and ascites, its role in patients with renal dysfunction is unclear. Few small studies on HRS indicate some clear benefit after TIPS[64,65]. A study examining non-transplantable cirrhotic (14 type 1 HRS and 17 type 2 HRS) patients showed renal function improved within two weeks after TIPS with improved mortality over the course of 18 mo[66]. A recent study utilizing UNOS demonstrated that patients on the LT list status-post TIPS procedure had a lower mortality rate compared to patients without TIPS[67]. This study hypothesized that the TIPS plays a role in promoting survival by improving nutritional status and preventing variceal bleeding, refractory ascites, and HRS. It is important to remember that TIPS can increase the risk of hepatic encephalopathy as well as liver failure in rare occasions[68].
Molecular absorbent recycling system (MARS) has the ability to remove both small- and medium-sized lipophilic toxins and may have a role in improving complications of liver disease such as hepatic encephalopathy and HRS. Multiple studies have shown MARS having the ability to reduce cholestatic parameters, improve mentation, as well as renal function especially in patients with a Model for End-Stage Liver Disease (MELD) between 20-29[69,70]. In 2002 a study showed when MARS was used there was improvement in mentation and hepatic encephalopathy in 14 out of 19 centers[71]. Interestingly, when MARS was directly compared to hemodiafiltration there was a decrease in Cr and bilirubin as well as a decrease in mortality at day 7[72]. Furthermore, a study looking at MARS use in the post-transplantation setting with HRS, HE, or intractable pruritis showed improvement in symptoms and laboratory findings[73]. However, none of these studies showed long term benefit in HRS patients including transplant free survival.
PREDICTORS OF MORTALITY IN PATIENTS WITH HRS
Yang et al[74] studied the predictors of mortality in type 1 HRS in a tertiary care center and formulated a time-dependent proportional hazards model. Contrary to other studies reporting on MELD score as predictor mortality, they found increased Cr by each point and total bilirubin levels during the admission increased mortality risk by 29% and 4%, respectively. Increasing albumin level during the admission showed its protective value[74].
Sanchez et al[75] looked at pre and peri-transplant predictors of renal dysfunction requiring either RRT or HD. This study looked at 724 LT patients where a clinical prediction model was constructed to assess the probability of requiring dialysis post-transplantation in a prospective manner. Pre-LT Cr > 1.9 mg/dL (OR = 3.57), pre-LT BUN > 27 mg/dL (OR = 2.68), ICU stay > 3 d (OR = 10.23), and MELD score > 21 (OR = 2.5) were significant[75]. Furthermore, changes in MELD scores (influenced by Cr and bilirubin) during the admission predict prognosis more so than the initial MELD[74]. A recent study was performed in attempts to assess renal impairment prior to overt HRS development by measuring renal arterial resistance indices (RI)[76]. Interestingly, RI was significantly higher in patients with ascites than those without ascites and may be an independent predictor of subsequent HRS development. Another study was able to show that “MAP responders” had improved response with better transplant-free survival in both the short-term and long-term settings[77]. However, these innovative modalities need further studies before being used in daily practice.
LT ALONE VS SIMULTANEOUS LIVER KIDNEY TRANSPLANT FOR HRS
Since the introduction of the MELD scoring system there has been an increase in the number of simultaneous liver-kidney transplants (SLKT). From 2002 to 2013, the percentage of SLKT has increased from 4.2% to 8.1%, respectively. The most recent recommendations for SLKT include: (1) eGFR of 30 mL/min or less for 4-8 wk; (2) proteinuria > 2 g/d; and (3) biopsy proven interstitial fibrosis or glomerulosclerosis (Figure 1)[78]. An unintentional by product of SLKT has been a decrease number of kidney donors available for end stage renal disease (ESRD) patients. There are numerous studies indicating we should have stricter criteria for allocating two grafts to one patient as well as a debate on duration of renal dysfunction and duration of RRT in the setting of SLKT. A recent study proposed raising the dialysis requirement to greater than 12 wk (rather than current recommendations of 4-8 wk) to increase the number of kidney transplantations available for ESRD patients[79]. Table 2 outlines the outcomes of studies comparing liver transplantation alone (LTA) alone vs SLKT in the setting of HRS. One study retrospectively looked at 69 LT patients with a pre-transplantation Cr ≥ 1.5 and found that duration of pre-transplantation RRT rather than cause of renal dysfunction was a predictor of 6- and 12-mo kidney function post-LTA[80]. Interestingly, earlier studies have shown mixed data in regards to the utility of SLKT in the setting of HRS. A 1997 UNOS study looked at 414 SLKT vs 2442 LTA with a Cr > 2.0 and found a 5 year survival of 62.2% for SLKT patients and 50.4% for LTA recipients, suggesting SLKT may be beneficial for HRS patients[81]. Furthermore, another study including local center and UNOS database (2002-2008) compared LTA vs SLKT in the setting of renal impairment. Diagnosis of HRS was presumptive in UNOS database and confirmed on the local data. UNOS data showed a survival benefit of SLKT over LTA for those patients with poor renal function, specifically those with HRS, whereas results of local center suggest otherwise[82].
Figure 1.
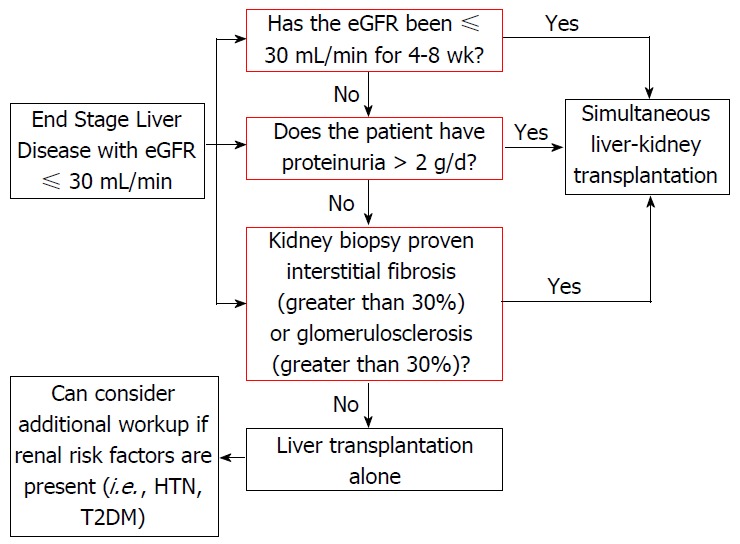
Algorithm for evaluating for simultaneous liver-kidney transplantation in a liver transplant candidate with renal dysfunction. Modified from Saxena et al[78]. eGFR: Estimation of glomerular filtration rate; T2DM: Type 2 diabetes mellitus; HTN: Hypertension.
Table 2.
Comparing outcomes measures between liver transplantation alone vs simultaneous liver kidney transplantation including graft and patient survival as well as need for renal replacement therapy
| Ref. | No. of LTA | No. of SLKT | Graft survival (LTA vs SLKT) | Patient survival (LTA vs SLKT) | Renal dysfunction post 1, 5 and 10 yr (LTA vs SLKT) | RRT post-transplantation (LTA vs SLKT) | Additional comments |
| Jeyarajah et al[81] | 2442 (Cr > 2.0, nationawide) | 29 (single center) + 414 (nationwide) | N/A | 5 yr survival nationwide (50.4% vs 62.2%) | N/A | N/A | Interestingly, single center study had increased better survival in LTA than SLKT group |
| Campbell et al[80] | 53 | 13 | N/A | N/A | 1 yr (1.4 mg/dL vs 1.5 mg/dL) | 2% vs 0% (at 12 mo) | Adjusting for baseline characteristics, SLKT patients had lower Cr than LTA at 12 mo (P = 0.01) |
| Ruiz et al[83] | 80 (all with HRS) | 98 (22 with HRS and 76 with primary renal disease) | 1 yr SLKT survival (liver: 76% and kidney: 76%) | 1 yr survival (66% LTA vs 72% SLKT) | N/A | Post-op dialysis: (89% LTA pts for median of 9 d vs 55% SLKT pts for median 2.5 d) | 1 yr acute kidney rejection in CLKT was 14% vs 23% in 5 yr LT cohort |
| Locke et al[84] | 19137 | 1032 | N/A | 1 yr survival for pts with ≥ 3 mo RRT: (70.8% LTA vs 84.5% SLKT) | N/A | N/A | Even after matched-control analysis, there was no benefit in SLKT cohort vs LTA cohort outside of aforementioned RRT |
| Mehrabi et al[85] (literature review) | N/A | 3536 | Cumulative 5 yr SLKT survival of both organs (60.9%) | Cumulative 5 yr survival 42.6% | N/A | N/A | It is concluded that there is no definitive evidence of better graft/patient survival in SKLT vs LTA |
| Chava et al[114] | N/A | 39 | 5 yr SLKT survival (liver: 73.7% and kidney: 70%) | 73.7% SLKT patient survival at 5 yr | N/A | N/A | 15 surviving patients (53.6%) had mild/moderate kidney dysfunction |
| Fong et al[82] | 2774 | 1501 | 5 yr survival (58.9% LTA vs 65.3%, SLKT, P < 0.001) | 5 yr survival (62.9% LTA vs 67.4% SLKT, P < 0.001) | 0% with severe renal dysfunction | N/A | Liver graft survival and patient survival was better in SLKT vs LTA group |
| Martin et al[88] 2012 | 66026 | 2327 | 15% decreased risk of graft loss with SLKT vs LTA (P = 0.02) | N/A | N/A | N/A | SLKT had higher graft survival rates than both KALT and LAKT |
| Sharma et al[86] | 2112 (received RRT within 90 d before LT) | N/A | N/A | 78% LTA survival at 6 mo (not associated with RRT duration) | N/A | 8.90% | Risk for non-recovery increased by 3.6%/day of pre-LT RRT |
| Catalano et al[89] | 74 | 37 | 10 yr survival (77% LTA vs 80% SLKT, P = 0.85) | 10 yr survival (79% LTA vs 86% SLKT, P = 0.56) | N/A | N/A | Acute rejection episodes involving the liver were less in SLKT vs LTA |
LTA: Liver transplantation alone; SLKT: Simultaneous liver kidney transplantationl; RRT: Renal replacement therapy; HRS: Hepatorenal syndrome; KALT: Kidney after liver transplantation; LAKT: Liver after kidney transplantation; N/A: Not available.
On the other hand, a small study showed that in patients with HRS, SLKT did not confer a survival advantage over LTA (1-year patient survival was 72% vs 66%, P-value = 0.88)[83]. A much larger 2006 UNOS study that compared 1032 SLKT to 19137 LTA patients showed no mortality difference for patients with HRS (1 year survival was 72% vs 66%) unless the patient was receiving HD for longer than 8 wk, with a dialysis duration of > 12 wk that was a significant predictor for long-term outcomes[84]. Furthermore, one meta-analysis looked at 3536 SLKT (between 1984 to 2008) and found that the cumulative 1, 2, 3 and 5-year patient survival were 84.9%, 52.8%, 45.4% and 42.6%. It was concluded that there was no definitive evidence of better graft or patient survival in the SLKT population when compared to the LTA given the difficulty discerning irreversible kidney function in liver transplant candidates[85]. Additionally, one study found the rate of renal non-recovery within 6 mo of LTA for 2112 patients who underwent RRT within 90 d of their transplantation was only 8.9%, with risk factors for non-recovery including age, T2DM, and duration of RRT[86]. Because of this limitation as well as selection biases, the true survival benefit of SLKT in candidates without ESRD remains unproved[87].
It appears that UNOS database studies have heterogeneous groups, including patients with renal impairment due to multiple reasons and hence a selection bias for patients with HRS. Single center studies have issue of small sample size. Nevertheless, chances of misclassification bias in small studies are less. These studies do not report added benefit of SLKT over LTA in patients with HRS, not on HD and duration of renal dysfunction < 8 wk.
Interestingly some studies address benefit of SKLT over LTA alone with respect to immune safety liver graft on kidney graft function due to immunogenic effect of liver. These studies justified SLKT over LTA for two additional reasons: (1) it is well documented there is significant decrease in graft rejection when a patient has a SLKT over an LTA (15% decreased reduction in graft loss); and (2) there is superior recipient and graft survival when compared to Kidney After LT or Liver After Kidney Transplantation[88,89]. Priority for allocation of kidneys to kidney-liver candidates follows the allocation priority for the non-renal organ. However, due to shortage of organ and justification of an equitable distribution of organ it is not possible to perform SLKT for this indication.
HRS AND POST-TRANSPLANT MANAGEMENT/OUTCOMES
Impact of HRS on outcomes of LT
The impact of LT on overall renal function has been well documented. Lafayette et al[90] looked at renal function in the pre-transplantation setting and studied 115 liver transplant recipients by arbitrarily dividing them based on serum Cr into two groups (group 1 with Cr > 1.0, group 2 with < 1.0); they showed that group 1 patients had significantly longer ICU stays, higher hospital charges, and a greatly increased mortality rate[90]. Patients with HRS tend to require longer hospitalizations, increased intensive care duration, and further dialysis in the post-op setting[91]. Interestingly, when comparing HRS vs ATN post-transplant outcomes it was found that ATN was associated with higher mortality at 1 year post-LT along with increased incidence of CKD (stage 4 or 5) when compared to HRS[31].
One of the first studies to address HRS in the post-operative setting was in reported in 1991 where Gonwa et al[92] found close to 10% of HRS patients developed ESRD post-transplant when compared to 0.8% of non-HRS patients (P < 0.005). However, a similar study revealed that while HRS patients were more likely to be dialyzed post-operatively, there was no difference between Cr levels at 24 wk between non-HRS vs HRS groups[93]. Park et al[94] also confirmed this concept in a study that yielded similar results in 1-year patient survival after LT in the HRS patients vs those without HRS (P = 0.37).
In regards to AKI in the post-LT setting, a large study looking at 1352 LT recipients found that 162 (12%) patients developed acute renal failure (ARF) within the first week. Type 2 HRS with GFR < 50 mL/min was reported to be one of major risk factor[95]. However, López Lago et al[96] also looked at HRS vs non-HRS patients who developed ARF in the post-LT setting but found no differences in 1 year mortality, need for RRT, or rejection.
Many studies have aimed to identify the role of GFR following transplantation in stratifying risk of kidney impairment. Sato et al[97] showed that an eGFR < 60 mL/min per 1.73 m2 during the first month post LT can be associated with increased rate of development of CKD, 2 years post-OLT. Interestingly, a recent study assessed 191 LT patients who underwent intense post LT GFR measurements (especially at 1 and 3 years). The study concluded that a low GFR (< 40 mL/min per 1.73 m2) at 1 year was associated with higher risk for late renal dysfunction[98]. Moreover, Longenecker et al[99] looked at the progression of GFR over 15 years post transplantation and found that eGFR < 60 mL/min per 1.73 m2 and type 2 diabetes at the time of transplantation were associated with increased rates of progression to ESRD. When discussing long-term requirement of RRT post-transplantation, one study assessed 208 LT recipients and found 5.8% of surviving patients required RRT at 3 mo. While there was no significant difference between underlying liver disease and immunosuppressive agents, patients who were on RRT at 3 mo were also on HD 2 years post-LT as well[100].
While the majority of studies seem to indicate HRS increases the risk for worse post-transplantation kidney function, there are certain exceptions found in the literature. One study looked at 419 LTA performed between 1995 to 2009 and found that MELD scoring system did not impact all-cause mortality in the post-transplantation setting; however, there was a 2-fold greater mortality risk if patients required the need for pre-transplant RRT and post-transplant kidney dysfunction[101].
Duration of pre-transplantation RRT and vasopressors for reversal of HRS
In regards to post-LT outcomes in patients with HRS who required vasopressor treatment one study compared 27 cases (triple therapy of octreotide, midodrine, and albumin) vs 16 controls (no vasopressor treatment) and found the GFR was similar at 1 mo (P = 0.61) and 1 year (P = 0.13)[58]. Moreover, 11 out of the 27 cases responded to triple therapy but there was no difference in GFR at 1 mo (P = 0.96) and 1 year (P = 0.48) between responders vs non-responders. A smaller study looked at 9 HRS patients on vasopressin vs 27 non-HRS patients and found there was no significant renal impairment between the two groups in regards to duration of hospitalizations, infections, or renal impairment post-transplantation[102]. These two studies are much different than the findings from Wong et al[103]; they found that patients without HRS reversal from triple therapy were found to have longer duration of pre-transplant dialysis and increase in post-transplant mortality[103].
One study assessed 253 living donor LT patients and compared survival between starting RRT in the pre-transplant setting vs post-transplant setting. It was found that the duration of RRT was significantly shorter in the RRT-pre group compared to the RRT-post group (5.3 ± 2.1 d vs 17.8 ± 14.1 d, P = 0.02) as well as higher graft survival (100% vs 51.9%, P < 0.01)[104].
How to manage immunosuppression in immediate post-LT period with HRS
Acute or chronic rejection has become more of a rarity with the current immunosuppression therapies[105]. However, calcineurin inhibitors (CNI) have significant nephrotoxic effects by inducing interstitial fibrosis, chronic microangiopathy, and tubular atrophy via increased extracellular matrix production, vasoconstriction, and cyclosporine induced apoptosis[11,106]. The landmark study in 1994 comparing tacrolimus vs cyclosporine showed that both were comparable in patient and graft survival; however, tacrolimus had substantially more adverse events, including nephrotoxicity, requiring discontinuation of the drug[107].
It is standard practice in majority of transplant centers to use different types of T-cell specific antibody induction in patients with post LT renal dysfunction. Commonly used agents are interleukin-2 receptor antagonists (daclizumab, or basiliximab) and polyclonal antibodies (rabbit anti-thymocyte globulin) based on center preference. Also it is practiced to use mycophenolate mofetil (MMF) and wait for improvement in kidney function post LT and introduce CNI.
Unfortunately, currently there is still no treatment for nephrotoxicity outside of dose reduction of current immunosuppressive regimen[108]. Patients who are more than 10 years post-transplant have a higher incidence of ESRD and chronic renal failure, which is related to increase in serum Cr at various stages post-operatively[109]. MMF has been used in situations where CNIs are held to improve renal function[110] but there exists greater risk for rejection when using MMF[111]. Cincinnati et al[112] show that combined MMF and low dose CNI therapy may actually promote tolerance, as this combination seems to be nephroprotective.
FURTHER RESEARCH AND CONCLUDING REMARKS
We aimed to review the literature on HRS in the setting of LT and focused on the critical issues that are barriers to improved outcomes. Many consistencies have remained as treatment options including albumin, vasoconstrictors, RRT, and eventual LT. One area that was not well addressed in our literature search was the utility of norepinephrine in the setting of type 1 HRS not responding to currently approved octreotide and terlipressin based pharmacotherapy.
While current guidelines for SLKT have been recently updated, there is still much debate regarding the utility of SLKT over LTA. Certain studies have shown improved graft and patient survival in the SLKT patient population, but the literature has not been consistent regarding long-term kidney benefit. This is a topic that we anticipate will need to be further explored given variable results seen at this time. Equity in organ allocation must be taken into consideration as SLKT unavoidably allocates multiple grafts to a single recipient and removes donor kidneys from the transplant pool otherwise meant for patients with primary renal disease.
Finally, in regards to post-transplantation kidney dysfunction an eGFR < 60 mL/min per 1.73 m2 seems to be associated with an increased risk of patients having long-term ESRD. While patients continue to have increased patient and graft survival rates, future studies may benefit from continuing to delineate risk factors that may result in post-transplant RRT.
ACKNOWLEDGMENTS
We would like to acknowledge Sarah E Ginier (Master of Library and Information Science Candidate May 2016) and Stephanie Schulte, MLIS (Associate Professor, Head, Research and Education Services) for their help in formulating the literature search for this review.
Footnotes
Conflict-of-interest statement: The authors do not have any disclosures to report.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: April 28, 2016
First decision: June 16, 2016
Article in press: July 18, 2016
P- Reviewer: Boin IFSF, Coban M S- Editor: Ji FF L- Editor: A E- Editor: Li D
References
- 1.Cárdenas A, Ginès P. Acute-on-chronic liver failure: the kidneys. Curr Opin Crit Care. 2011;17:184–189. doi: 10.1097/MCC.0b013e328344b3da. [DOI] [PubMed] [Google Scholar]
- 2.Biancofiore G, Davis CL. Renal dysfunction in the perioperative liver transplant period. Curr Opin Organ Transplant. 2008;13:291–297. doi: 10.1097/MOT.0b013e328300a058. [DOI] [PubMed] [Google Scholar]
- 3.Pipili C, Cholongitas E. Renal dysfunction in patients with cirrhosis: Where do we stand? World J Gastrointest Pharmacol Ther. 2014;5:156–168. doi: 10.4292/wjgpt.v5.i3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allegretti AS, Ortiz G, Wenger J, Deferio JJ, Wibecan J, Kalim S, Tamez H, Chung RT, Karumanchi SA, Thadhani RI. Prognosis of Acute Kidney Injury and Hepatorenal Syndrome in Patients with Cirrhosis: A Prospective Cohort Study. Int J Nephrol. 2015;2015:108139. doi: 10.1155/2015/108139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGuire BM, Julian BA, Bynon JS, Cook WJ, King SJ, Curtis JJ, Accortt NA, Eckhoff DE. Brief communication: Glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation. Ann Intern Med. 2006;144:735–741. doi: 10.7326/0003-4819-144-10-200605160-00007. [DOI] [PubMed] [Google Scholar]
- 6.Davis CL, Feng S, Sung R, Wong F, Goodrich NP, Melton LB, Reddy KR, Guidinger MK, Wilkinson A, Lake J. Simultaneous liver-kidney transplantation: evaluation to decision making. Am J Transplant. 2007;7:1702–1709. doi: 10.1111/j.1600-6143.2007.01856.x. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064–2077. doi: 10.1002/hep.22605. [DOI] [PubMed] [Google Scholar]
- 8.Baraldi O, Valentini C, Donati G, Comai G, Cuna V, Capelli I, Angelini ML, Moretti MI, Angeletti A, Piscaglia F, et al. Hepatorenal syndrome: Update on diagnosis and treatment. World J Nephrol. 2015;4:511–520. doi: 10.5527/wjn.v4.i5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davenport A, Cholongitas E, Xirouchakis E, Burroughs AK. Pitfalls in assessing renal function in patients with cirrhosis--potential inequity for access to treatment of hepatorenal failure and liver transplantation. Nephrol Dial Transplant. 2011;26:2735–2742. doi: 10.1093/ndt/gfr354. [DOI] [PubMed] [Google Scholar]
- 10.Cocchetto DM, Tschanz C, Bjornsson TD. Decreased rate of creatinine production in patients with hepatic disease: implications for estimation of creatinine clearance. Ther Drug Monit. 1983;5:161–168. doi: 10.1097/00007691-198306000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Francoz C, Glotz D, Moreau R, Durand F. The evaluation of renal function and disease in patients with cirrhosis. J Hepatol. 2010;52:605–613. doi: 10.1016/j.jhep.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Moylan CA, Brady CW, Johnson JL, Smith AD, Tuttle-Newhall JE, Muir AJ. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300:2371–2378. doi: 10.1001/jama.2008.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Perrone RD, Madias NE. Serum creatinine and renal function. Annu Rev Med. 1988;39:465–490. doi: 10.1146/annurev.me.39.020188.002341. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Sterner G, Frennby B, Mansson S, Nyman U, Van Westen D, Almén T. Determining ‘true’ glomerular filtration rate in healthy adults using infusion of inulin and comparing it with values obtained using other clearance techniques or prediction equations. Scand J Urol Nephrol. 2008;42:278–285. doi: 10.1080/00365590701701806. [DOI] [PubMed] [Google Scholar]
- 16.Cruz DN, Bagshaw SM, Maisel A, Lewington A, Thadhani R, Chakravarthi R, Murray PT, Mehta RL, Chawla LS. Use of biomarkers to assess prognosis and guide management of patients with acute kidney injury. Contrib Nephrol. 2013;182:45–64. doi: 10.1159/000349965. [DOI] [PubMed] [Google Scholar]
- 17.Qasem AA, Farag SE, Hamed E, Emara M, Bihery A, Pasha H. Urinary biomarkers of acute kidney injury in patients with liver cirrhosis. ISRN Nephrol. 2014;2014:376795. doi: 10.1155/2014/376795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aberg F, Lempinen M, Hollmén M, Nordin A, Mäkisalo H, Isoniemi H. Neutrophil gelatinase-associated lipocalin associated with irreversibility of pre-liver transplant kidney dysfunction. Clin Transplant. 2014;28:869–876. doi: 10.1111/ctr.12394. [DOI] [PubMed] [Google Scholar]
- 19.Verna EC, Brown RS, Farrand E, Pichardo EM, Forster CS, Sola-Del Valle DA, Adkins SH, Sise ME, Oliver JA, Radhakrishnan J, et al. Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci. 2012;57:2362–2370. doi: 10.1007/s10620-012-2180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo YS, Jung ES, An H, Kim JH, Jung YK, Kim JH, Yim HJ, Yeon JE, Byun KS, Kim CD, et al. Serum cystatin C level is a good prognostic marker in patients with cirrhotic ascites and normal serum creatinine levels. Liver Int. 2009;29:1521–1527. doi: 10.1111/j.1478-3231.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- 21.Sharawey MA, Shawky EM, Ali LH, Mohammed AA, Hassan HA, Fouad YM. Cystatin C: a predictor of hepatorenal syndrome in patients with liver cirrhosis. Hepatol Int. 2011;5:927–933. doi: 10.1007/s12072-011-9266-y. [DOI] [PubMed] [Google Scholar]
- 22.Barbano B, Sardo L, Gigante A, Gasperini ML, Liberatori M, Giraldi GD, Lacanna A, Amoroso A, Cianci R. Pathophysiology, diagnosis and clinical management of hepatorenal syndrome: from classic to new drugs. Curr Vasc Pharmacol. 2014;12:125–135. doi: 10.2174/157016111201140327163930. [DOI] [PubMed] [Google Scholar]
- 23.Bataller R, Ginès P, Arroyo V, Rodés J. Hepatorenal syndrome. Clin Liver Dis. 2000;4:487–507. doi: 10.1016/s1089-3261(05)70120-3. [DOI] [PubMed] [Google Scholar]
- 24.Mijac D, Kezić A, Stojimirović B. [Hepatorenal syndrome] Srp Arh Celok Lek. 2007;135:98–104. [PubMed] [Google Scholar]
- 25.Cárdenas A, Ginès P. Therapy insight: Management of hepatorenal syndrome. Nat Clin Pract Gastroenterol Hepatol. 2006;3:338–348. doi: 10.1038/ncpgasthep0517. [DOI] [PubMed] [Google Scholar]
- 26.Alessandria C, Ozdogan O, Guevara M, Restuccia T, Jiménez W, Arroyo V, Rodés J, Ginès P. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology. 2005;41:1282–1289. doi: 10.1002/hep.20687. [DOI] [PubMed] [Google Scholar]
- 27.Thomas ME, Blaine C, Dawnay A, Devonald MA, Ftouh S, Laing C, Latchem S, Lewington A, Milford DV, Ostermann M. The definition of acute kidney injury and its use in practice. Kidney Int. 2015;87:62–73. doi: 10.1038/ki.2014.328. [DOI] [PubMed] [Google Scholar]
- 28.Hartleb M, Gutkowski K. Kidneys in chronic liver diseases. World J Gastroenterol. 2012;18:3035–3049. doi: 10.3748/wjg.v18.i24.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fagundes C, Barreto R, Guevara M, Garcia E, Solà E, Rodríguez E, Graupera I, Ariza X, Pereira G, Alfaro I, et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. 2013;59:474–481. doi: 10.1016/j.jhep.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 30.Piano S, Rosi S, Maresio G, Fasolato S, Cavallin M, Romano A, Morando F, Gola E, Frigo AC, Gatta A, et al. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013;59:482–489. doi: 10.1016/j.jhep.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 31.Nadim MK, Genyk YS, Tokin C, Fieber J, Ananthapanyasut W, Ye W, Selby R. Impact of the etiology of acute kidney injury on outcomes following liver transplantation: acute tubular necrosis versus hepatorenal syndrome. Liver Transpl. 2012;18:539–548. doi: 10.1002/lt.23384. [DOI] [PubMed] [Google Scholar]
- 32.Junghare M, Ibrahim HN. Not all types of acute kidney injury are equal in the setting of liver transplantation. Liver Transpl. 2012;18:507–508. doi: 10.1002/lt.23428. [DOI] [PubMed] [Google Scholar]
- 33.Russ KB, Stevens TM, Singal AK. Acute Kidney Injury in Patients with Cirrhosis. J Clin Transl Hepatol. 2015;3:195–204. doi: 10.14218/JCTH.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nair S, Verma S, Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002;35:1179–1185. doi: 10.1053/jhep.2002.33160. [DOI] [PubMed] [Google Scholar]
- 35.Martín-Llahí M, Guevara M, Torre A, Fagundes C, Restuccia T, Gilabert R, Solá E, Pereira G, Marinelli M, Pavesi M, et al. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology. 2011;140:488–496.e4. doi: 10.1053/j.gastro.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 36.Ginès P, Guevara M, Arroyo V, Rodés J. Hepatorenal syndrome. Lancet. 2003;362:1819–1827. doi: 10.1016/S0140-6736(03)14903-3. [DOI] [PubMed] [Google Scholar]
- 37.Wong LP, Blackley MP, Andreoni KA, Chin H, Falk RJ, Klemmer PJ. Survival of liver transplant candidates with acute renal failure receiving renal replacement therapy. Kidney Int. 2005;68:362–370. doi: 10.1111/j.1523-1755.2005.00408.x. [DOI] [PubMed] [Google Scholar]
- 38.EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Arroyo V, Fernández J. Management of hepatorenal syndrome in patients with cirrhosis. Nat Rev Nephrol. 2011;7:517–526. doi: 10.1038/nrneph.2011.96. [DOI] [PubMed] [Google Scholar]
- 40.Angeli P, Morando F, Cavallin M, Piano S. Hepatorenal syndrome. Contrib Nephrol. 2011;174:46–55. doi: 10.1159/000329235. [DOI] [PubMed] [Google Scholar]
- 41.Cavallin M, Fasolato S, Marenco S, Piano S, Tonon M, Angeli P. The Treatment of Hepatorenal Syndrome. Dig Dis. 2015;33:548–554. doi: 10.1159/000375346. [DOI] [PubMed] [Google Scholar]
- 42.Danalioglu A, Cakaloglu Y, Karaca C, Aksoy N, Akyuz F, Ozdil S, Demir K, Besisik F, Boztas G, Mungan Z, et al. Terlipressin and albumin combination treatment in hepatorenal syndrome. Hepatogastroenterology. 2003;50 Suppl 2:ccciii–cccccv. [PubMed] [Google Scholar]
- 43.Cavallin M, Piano S, Romano A, Fasolato S, Frigo AC, Benetti G, Gola E, Morando F, Stanco M, Rosi S, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: A randomized controlled study. Hepatology. 2016;63:983–992. doi: 10.1002/hep.28396. [DOI] [PubMed] [Google Scholar]
- 44.Boyer TD, Sanyal AJ, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, Gülberg V, Sigal S, Bexon AS, Teuber P. Impact of liver transplantation on the survival of patients treated for hepatorenal syndrome type 1. Liver Transpl. 2011;17:1328–1332. doi: 10.1002/lt.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sagi SV, Mittal S, Kasturi KS, Sood GK. Terlipressin therapy for reversal of type 1 hepatorenal syndrome: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2010;25:880–885. doi: 10.1111/j.1440-1746.2009.06132.x. [DOI] [PubMed] [Google Scholar]
- 46.Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, Blei A, Gülberg V, Sigal S, Teuber P. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360–1368. doi: 10.1053/j.gastro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Kalckreuth V, Glowa F, Geibler M, Lohse AW, Denzer UW. Terlipressin in 30 patients with hepatorenal syndrome: results of a retrospective study. Z Gastroenterol. 2009;47:21–26. doi: 10.1055/s-0028-1109084. [DOI] [PubMed] [Google Scholar]
- 48.Fabrizi F, Aghemo A, Messa P. Hepatorenal syndrome and novel advances in its management. Kidney Blood Press Res. 2013;37:588–601. doi: 10.1159/000355739. [DOI] [PubMed] [Google Scholar]
- 49.Halimi C, Bonnard P, Bernard B, Mathurin P, Mofredj A, di Martino V, Demontis R, Henry-Biabaud E, Fievet P, Opolon P, et al. Effect of terlipressin (Glypressin) on hepatorenal syndrome in cirrhotic patients: results of a multicentre pilot study. Eur J Gastroenterol Hepatol. 2002;14:153–158. doi: 10.1097/00042737-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Heidemann J, Bartels C, Berssenbrügge C, Schmidt H, Meister T. Hepatorenal syndrome: outcome of response to therapy and predictors of survival. Gastroenterol Res Pract. 2015;2015:457613. doi: 10.1155/2015/457613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hinz M, Wree A, Jochum C, Bechmann LP, Saner F, Gerbes AL, Gerken G, Canbay A. High age and low sodium urine concentration are associated with poor survival in patients with hepatorenal syndrome. Ann Hepatol. 2013;12:92–99. [PubMed] [Google Scholar]
- 52.Alessandria C, Venon WD, Marzano A, Barletti C, Fadda M, Rizzetto M. Renal failure in cirrhotic patients: role of terlipressin in clinical approach to hepatorenal syndrome type 2. Eur J Gastroenterol Hepatol. 2002;14:1363–1368. doi: 10.1097/00042737-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez E, Henrique Pereira G, Solà E, Elia C, Barreto R, Pose E, Colmenero J, Fernandez J, Navasa M, Arroyo V, et al. Treatment of type 2 hepatorenal syndrome in patients awaiting transplantation: Effects on kidney function and transplantation outcomes. Liver Transpl. 2015;21:1347–1354. doi: 10.1002/lt.24210. [DOI] [PubMed] [Google Scholar]
- 54.Testro AG, Wongseelashote S, Angus PW, Gow PJ. Long-term outcome of patients treated with terlipressin for types 1 and 2 hepatorenal syndrome. J Gastroenterol Hepatol. 2008;23:1535–1540. doi: 10.1111/j.1440-1746.2007.05176.x. [DOI] [PubMed] [Google Scholar]
- 55.Tan HK, Marquez M, Wong F, Renner EL. Pretransplant Type 2 Hepatorenal Syndrome Is Associated With Persistently Impaired Renal Function After Liver Transplantation. Transplantation. 2015;99:1441–1446. doi: 10.1097/TP.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 56.Kiser TH, Fish DN, Obritsch MD, Jung R, MacLaren R, Parikh CR. Vasopressin, not octreotide, may be beneficial in the treatment of hepatorenal syndrome: a retrospective study. Nephrol Dial Transplant. 2005;20:1813–1820. doi: 10.1093/ndt/gfh930. [DOI] [PubMed] [Google Scholar]
- 57.Skagen C, Einstein M, Lucey MR, Said A. Combination treatment with octreotide, midodrine, and albumin improves survival in patients with type 1 and type 2 hepatorenal syndrome. J Clin Gastroenterol. 2009;43:680–685. doi: 10.1097/MCG.0b013e318188947c. [DOI] [PubMed] [Google Scholar]
- 58.Rice JP, Skagen C, Said A. Liver transplant outcomes for patients with hepatorenal syndrome treated with pretransplant vasoconstrictors and albumin. Transplantation. 2011;91:1141–1147. doi: 10.1097/TP.0b013e31821690bf. [DOI] [PubMed] [Google Scholar]
- 59.Mulkay JP, Louis H, Donckier V, Bourgeois N, Adler M, Deviere J, Le Moine O. Long-term terlipressin administration improves renal function in cirrhotic patients with type 1 hepatorenal syndrome: a pilot study. Acta Gastroenterol Belg. 2001;64:15–19. [PubMed] [Google Scholar]
- 60.Alessandria C, Ottobrelli A, Debernardi-Venon W, Todros L, Cerenzia MT, Martini S, Balzola F, Morgando A, Rizzetto M, Marzano A. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. J Hepatol. 2007;47:499–505. doi: 10.1016/j.jhep.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 61.Nassar Junior AP, Farias AQ, D’ Albuquerque LA, Carrilho FJ, Malbouisson LM. Terlipressin versus norepinephrine in the treatment of hepatorenal syndrome: a systematic review and meta-analysis. PLoS One. 2014;9:e107466. doi: 10.1371/journal.pone.0107466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cavallin M, Kamath PS, Merli M, Fasolato S, Toniutto P, Salerno F, Bernardi M, Romanelli RG, Colletta C, Salinas F, et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: A randomized trial. Hepatology. 2015;62:567–574. doi: 10.1002/hep.27709. [DOI] [PubMed] [Google Scholar]
- 63.Caraceni P, Santi L, Mirici F, Montanari G, Bevilacqua V, Pinna AD, Bernardi M. Long-term treatment of hepatorenal syndrome as a bridge to liver transplantation. Dig Liver Dis. 2011;43:242–245. doi: 10.1016/j.dld.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 64.Guevara M, Ginès P, Bandi JC, Gilabert R, Sort P, Jiménez W, Garcia-Pagan JC, Bosch J, Arroyo V, Rodés J. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: effects on renal function and vasoactive systems. Hepatology. 1998;28:416–422. doi: 10.1002/hep.510280219. [DOI] [PubMed] [Google Scholar]
- 65.Brensing KA, Textor J, Strunk H, Klehr HU, Schild H, Sauerbruch T. Transjugular intrahepatic portosystemic stent-shunt for hepatorenal syndrome. Lancet. 1997;349:697–698. doi: 10.1016/s0140-6736(97)24010-9. [DOI] [PubMed] [Google Scholar]
- 66.Brensing KA, Textor J, Perz J, Schiedermaier P, Raab P, Strunk H, Klehr HU, Kramer HJ, Spengler U, Schild H, et al. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut. 2000;47:288–295. doi: 10.1136/gut.47.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berry K, Lerrigo R, Liou IW, Ioannou GN. Association Between Transjugular Intrahepatic Portosystemic Shunt and Survival in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2016;14:118–123. doi: 10.1016/j.cgh.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 68.Brown RS, Lake JR. Transjugular intrahepatic portosystemic shunt as a form of treatment for portal hypertension: indications and contraindications. Adv Intern Med. 1997;42:485–504. [PubMed] [Google Scholar]
- 69.Di Campli C, Santoro MC, Gaspari R, Merra G, Zileri Dal Verme L, Zocco MA, Piscaglia AC, Di Gioacchino G, Novi M, Santoliquido A, et al. Catholic university experience with molecular adsorbent recycling system in patients with severe liver failure. Transplant Proc. 2005;37:2547–2550. doi: 10.1016/j.transproceed.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 70.Mitzner SR, Klammt S, Peszynski P, Hickstein H, Korten G, Stange J, Schmidt R. Improvement of multiple organ functions in hepatorenal syndrome during albumin dialysis with the molecular adsorbent recirculating system. Ther Apher. 2001;5:417–422. doi: 10.1046/j.1526-0968.2001.00388.x. [DOI] [PubMed] [Google Scholar]
- 71.Stange J, Hassanein TI, Mehta R, Mitzner SR, Bartlett RH. The molecular adsorbents recycling system as a liver support system based on albumin dialysis: a summary of preclinical investigations, prospective, randomized, controlled clinical trial, and clinical experience from 19 centers. Artif Organs. 2002;26:103–110. doi: 10.1046/j.1525-1594.2002.06822.x. [DOI] [PubMed] [Google Scholar]
- 72.Mitzner SR, Stange J, Klammt S, Risler T, Erley CM, Bader BD, Berger ED, Lauchart W, Peszynski P, Freytag J, et al. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl. 2000;6:277–286. doi: 10.1002/lt.500060326. [DOI] [PubMed] [Google Scholar]
- 73.Gaspari R, Cavaliere F, Sollazzi L, Perilli V, Melchionda I, Agnes S, Gasbarrini A, Avolio AW. Molecular adsorbent recirculating system (Mars) in patients with primary nonfunction and other causes of graft dysfunction after liver transplantation in the era of extended criteria donor organs. Transplant Proc. 2009;41:253–258. doi: 10.1016/j.transproceed.2008.10.066. [DOI] [PubMed] [Google Scholar]
- 74.Yang YW, Wu CH, Hu RH, Ho MC, Tsai MK, Wu YM, Lee PH. Longitudinal assessment of prognostic factors for patients with hepatorenal syndrome in a tertiary center. Hepatol Int. 2010;4:507–510. doi: 10.1007/s12072-010-9180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanchez EQ, Gonwa TA, Levy MF, Goldstein RM, Mai ML, Hays SR, Melton LB, Saracino G, Klintmalm GB. Preoperative and perioperative predictors of the need for renal replacement therapy after orthotopic liver transplantation. Transplantation. 2004;78:1048–1054. doi: 10.1097/01.tp.0000137176.95730.5b. [DOI] [PubMed] [Google Scholar]
- 76.Goyal S, Dixit VK, Jain AK, Shukla RC, Ghosh J, Kumar V. Intrarenal resistance index (RI) as a predictor of early renal impairment in patients with liver cirrhosis. Trop Gastroenterol. 2013;34:235–239. doi: 10.7869/tg.140. [DOI] [PubMed] [Google Scholar]
- 77.Maddukuri G, Cai CX, Munigala S, Mohammadi F, Zhang Z. Targeting an early and substantial increase in mean arterial pressure is critical in the management of type 1 hepatorenal syndrome: a combined retrospective and pilot study. Dig Dis Sci. 2014;59:471–481. doi: 10.1007/s10620-013-2899-z. [DOI] [PubMed] [Google Scholar]
- 78.Saxena V, Lai JC. Kidney Failure and Liver Allocation: Current Practices and Potential Improvements. Adv Chronic Kidney Dis. 2015;22:391–398. doi: 10.1053/j.ackd.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang Y, Gallon L, Shetty K, Chang Y, Jay C, Levitsky J, Ho B, Baker T, Ladner D, Friedewald J, et al. Simulation modeling of the impact of proposed new simultaneous liver and kidney transplantation policies. Transplantation. 2015;99:424–430. doi: 10.1097/TP.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Campbell MS, Kotlyar DS, Brensinger CM, Lewis JD, Shetty K, Bloom RD, Markmann JF, Olthoff KM, Shaked A, Reddy KR. Renal function after orthotopic liver transplantation is predicted by duration of pretransplantation creatinine elevation. Liver Transpl. 2005;11:1048–1055. doi: 10.1002/lt.20445. [DOI] [PubMed] [Google Scholar]
- 81.Jeyarajah DR, Gonwa TA, McBride M, Testa G, Abbasoglu O, Husberg BS, Levy MF, Goldstein RM, Klintmalm GB. Hepatorenal syndrome: combined liver kidney transplants versus isolated liver transplant. Transplantation. 1997;64:1760–1765. doi: 10.1097/00007890-199712270-00024. [DOI] [PubMed] [Google Scholar]
- 82.Fong TL, Khemichian S, Shah T, Hutchinson IV, Cho YW. Combined liver-kidney transplantation is preferable to liver transplant alone for cirrhotic patients with renal failure. Transplantation. 2012;94:411–416. doi: 10.1097/TP.0b013e3182590d6b. [DOI] [PubMed] [Google Scholar]
- 83.Ruiz R, Kunitake H, Wilkinson AH, Danovitch GM, Farmer DG, Ghobrial RM, Yersiz H, Hiatt JR, Busuttil RW. Long-term analysis of combined liver and kidney transplantation at a single center. Arch Surg. 2006;141:735–741; discussion 741-742. doi: 10.1001/archsurg.141.8.735. [DOI] [PubMed] [Google Scholar]
- 84.Locke JE, Warren DS, Singer AL, Segev DL, Simpkins CE, Maley WR, Montgomery RA, Danovitch G, Cameron AM. Declining outcomes in simultaneous liver-kidney transplantation in the MELD era: ineffective usage of renal allografts. Transplantation. 2008;85:935–942. doi: 10.1097/TP.0b013e318168476d. [DOI] [PubMed] [Google Scholar]
- 85.Mehrabi A, Fonouni H, Ayoub E, Rahbari NN, Müller SA, Morath Ch, Seckinger J, Sadeghi M, Golriz M, Esmaeilzadeh M, Hillebrand N, Weitz J, Zeier M, Büchler MW, Schmidt J, Schmied BM. A single center experience of combined liver kidney transplantation. Clin Transplant. 2009;23 Suppl 21:102–114. doi: 10.1111/j.1399-0012.2009.01146.x. [DOI] [PubMed] [Google Scholar]
- 86.Sharma P, Goodrich NP, Zhang M, Guidinger MK, Schaubel DE, Merion RM. Short-term pretransplant renal replacement therapy and renal nonrecovery after liver transplantation alone. Clin J Am Soc Nephrol. 2013;8:1135–1142. doi: 10.2215/CJN.09600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sung RS, Wiseman AC. Simultaneous Liver-Kidney Transplant: Too Many or Just Enough? Adv Chronic Kidney Dis. 2015;22:399–403. doi: 10.1053/j.ackd.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 88.Martin EF, Huang J, Xiang Q, Klein JP, Bajaj J, Saeian K. Recipient survival and graft survival are not diminished by simultaneous liver-kidney transplantation: an analysis of the united network for organ sharing database. Liver Transpl. 2012;18:914–929. doi: 10.1002/lt.23440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Catalano G, Tandoi F, Mazza E, Simonato F, Tognarelli G, Biancone L, Lupo F, Romagnoli R, Salizzoni M. Simultaneous Liver-Kidney Transplantation in Adults: A Single-center Experience Comparing Results With Isolated Liver Transplantation. Transplant Proc. 2015;47:2156–2158. doi: 10.1016/j.transproceed.2014.11.073. [DOI] [PubMed] [Google Scholar]
- 90.Lafayette RA, Paré G, Schmid CH, King AJ, Rohrer RJ, Nasraway SA. Pretransplant renal dysfunction predicts poorer outcome in liver transplantation. Clin Nephrol. 1997;48:159–164. [PubMed] [Google Scholar]
- 91.Gonwa TA, Klintmalm GB, Levy M, Jennings LS, Goldstein RM, Husberg BS. Impact of pretransplant renal function on survival after liver transplantation. Transplantation. 1995;59:361–365. [PubMed] [Google Scholar]
- 92.Gonwa TA, Morris CA, Goldstein RM, Husberg BS, Klintmalm GB. Long-term survival and renal function following liver transplantation in patients with and without hepatorenal syndrome--experience in 300 patients. Transplantation. 1991;51:428–430. doi: 10.1097/00007890-199102000-00030. [DOI] [PubMed] [Google Scholar]
- 93.Seu P, Wilkinson AH, Shaked A, Busuttil RW. The hepatorenal syndrome in liver transplant recipients. Am Surg. 1991;57:806–809. [PubMed] [Google Scholar]
- 94.Park I, Moon E, Hwang JA, Yu S, Kim BW, Wang HJ, Shin GT, Kim H. Does hepatorenal syndrome affect the result of liver transplantation? Clinical observations. Transplant Proc. 2010;42:2563–2566. doi: 10.1016/j.transproceed.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 95.Junge G, Schewior LV, Kohler S, Neuhaus R, Langrehr JM, Tullius S, Kahl A, Frei U, Neuhaus P. Acute renal failure after liver transplantation: incidence, etiology, therapy, and outcome. Transplant Proc. 2006;38:723–724. doi: 10.1016/j.transproceed.2006.01.074. [DOI] [PubMed] [Google Scholar]
- 96.López Lago AM, Fernández Villanueva J, García Acuña JM, Paz ES, Vizoso EF, Pérez EV. Evolution of hepatorenal syndrome after orthotopic liver transplantation: comparative analysis with patients who developed acute renal failure in the early postoperative period of liver transplantation. Transplant Proc. 2007;39:2318–2319. doi: 10.1016/j.transproceed.2007.07.070. [DOI] [PubMed] [Google Scholar]
- 97.Sato K, Kawagishi N, Fujimori K, Ohuchi N, Satomi S. Renal function status in liver transplant patients in the first month post-transplant is associated with progressive chronic kidney disease. Hepatol Res. 2015;45:220–227. doi: 10.1111/hepr.12339. [DOI] [PubMed] [Google Scholar]
- 98.Cohen AJ, Stegall MD, Rosen CB, Wiesner RH, Leung N, Kremers WK, Zein NN. Chronic renal dysfunction late after liver transplantation. Liver Transpl. 2002;8:916–921. doi: 10.1053/jlts.2002.35668. [DOI] [PubMed] [Google Scholar]
- 99.Longenecker JC, Estrella MM, Segev DL, Atta MG. Patterns of Kidney Function Before and After Orthotopic Liver Transplant: Associations With Length of Hospital Stay, Progression to End-Stage Renal Disease, and Mortality. Transplantation. 2015;99:2556–2564. doi: 10.1097/TP.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 100.Weigand K, Bauer E, Encke J, Schmidt J, Stremmel W, Schwenger V. Prognostic value of standard parameters as predictors for long-term renal replacement therapy after liver transplantation. Nephron Clin Pract. 2011;119:c342–c347. doi: 10.1159/000331072. [DOI] [PubMed] [Google Scholar]
- 101.Sethi A, Estrella MM, Ugarte R, Atta MG. Kidney function and mortality post-liver transplant in the Model for End-Stage Liver Disease era. Int J Nephrol Renovasc Dis. 2011;4:139–144. doi: 10.2147/IJNRD.S24812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Restuccia T, Ortega R, Guevara M, Ginès P, Alessandria C, Ozdogan O, Navasa M, Rimola A, Garcia-Valdecasas JC, Arroyo V, et al. Effects of treatment of hepatorenal syndrome before transplantation on posttransplantation outcome. A case-control study. J Hepatol. 2004;40:140–146. doi: 10.1016/j.jhep.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 103.Wong F, Leung W, Al Beshir M, Marquez M, Renner EL. Outcomes of patients with cirrhosis and hepatorenal syndrome type 1 treated with liver transplantation. Liver Transpl. 2015;21:300–307. doi: 10.1002/lt.24049. [DOI] [PubMed] [Google Scholar]
- 104.Ikegami T, Shirabe K, Soejima Y, Taketomi A, Yoshizumi T, Uchiyama H, Harada N, Maehara Y. The impact of renal replacement therapy before or after living donor liver transplantation. Clin Transplant. 2012;26:143–148. doi: 10.1111/j.1399-0012.2011.01450.x. [DOI] [PubMed] [Google Scholar]
- 105.Jain A, Reyes J, Kashyap R, Dodson SF, Demetris AJ, Ruppert K, Abu-Elmagd K, Marsh W, Madariaga J, Mazariegos G, et al. Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann Surg. 2000;232:490–500. doi: 10.1097/00000658-200010000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Davis CL, Gonwa TA, Wilkinson AH. Pathophysiology of renal disease associated with liver disorders: implications for liver transplantation. Part I. Liver Transpl. 2002;8:91–109. doi: 10.1053/jlts.2002.31516. [DOI] [PubMed] [Google Scholar]
- 107.A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. The U.S. Multicenter FK506 Liver Study Group. N Engl J Med. 1994;331:1110–1115. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 108.Distant DA, Gonwa TA. The kidney in liver transplantation. J Am Soc Nephrol. 1993;4:129–136. doi: 10.1681/ASN.V42129. [DOI] [PubMed] [Google Scholar]
- 109.Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, Klintmalm GB. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation. 2001;72:1934–1939. doi: 10.1097/00007890-200112270-00012. [DOI] [PubMed] [Google Scholar]
- 110.de Mattos AM, Olyaei AJ, Bennett WM. Nephrotoxicity of immunosuppressive drugs: long-term consequences and challenges for the future. Am J Kidney Dis. 2000;35:333–346. doi: 10.1016/s0272-6386(00)70348-9. [DOI] [PubMed] [Google Scholar]
- 111.Schlitt HJ, Barkmann A, Böker KH, Schmidt HH, Emmanouilidis N, Rosenau J, Bahr MJ, Tusch G, Manns MP, Nashan B, et al. Replacement of calcineurin inhibitors with mycophenolate mofetil in liver-transplant patients with renal dysfunction: a randomised controlled study. Lancet. 2001;357:587–591. doi: 10.1016/s0140-6736(00)04055-1. [DOI] [PubMed] [Google Scholar]
- 112.Cicinnati VR, Yu Z, Klein CG, Sotiropoulos GC, Saner F, Malagó M, Frilling A, Gerken G, Broelsch CE, Beckebaum S. Clinical trial: switch to combined mycophenolate mofetil and minimal dose calcineurin inhibitor in stable liver transplant patients--assessment of renal and allograft function, cardiovascular risk factors and immune monitoring. Aliment Pharmacol Ther. 2007;26:1195–1208. doi: 10.1111/j.1365-2036.2007.03466.x. [DOI] [PubMed] [Google Scholar]
- 113.Hadengue A, Gadano A, Moreau R, Giostra E, Durand F, Valla D, Erlinger S, Lebrec D. Beneficial effects of the 2-day administration of terlipressin in patients with cirrhosis and hepatorenal syndrome. J Hepatol. 1998;29:565–570. doi: 10.1016/s0168-8278(98)80151-7. [DOI] [PubMed] [Google Scholar]
- 114.Chava SP, Singh B, Stangou A, Battula N, Bowles M, O’Grady J, Rela M, Heaton ND. Simultaneous combined liver and kidney transplantation: a single center experience. Clin Transplant. 2010;24:E62–E68. doi: 10.1111/j.1399-0012.2010.01168.x. [DOI] [PubMed] [Google Scholar]


