Figure 1.
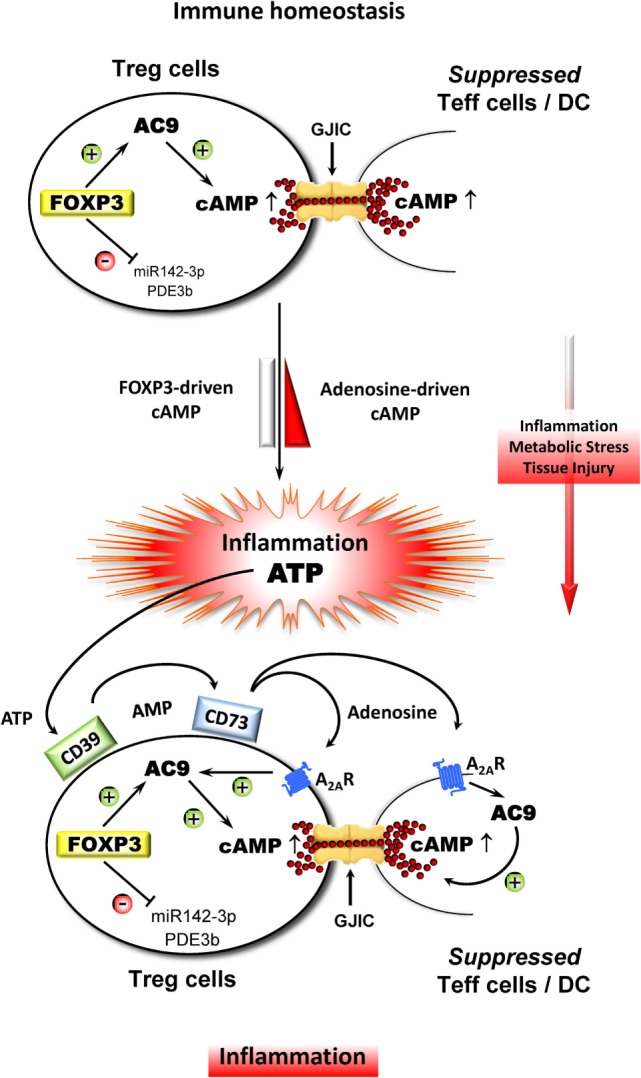
Adenosine strongly improves the suppressive influence of Treg cell-derived cAMP in the course of inflammation. During immune homeostasis, Treg cells stabilize the ITN with the aid of endogenous cAMP that is driven by FOXP3, which indirectly upregulates adenylate cylase 9 (AC9) through the inhibition of miR142-3p and which concomitantly downregulates cAMP-degrading phosphodiesterase 3b (PDE3b). As a result, Treg cells contain comparatively high amounts of cAMP leading to the suppression of Teff cells and DC via gap junctional intercellular communication (GJIC). Inflammation in combination with metabolic stress and tissue injury results in a massive release of ATP, which represents a powerful danger signal that serves as an additional local inflammatory booster that bears the risk of collateral damage by uncontrollable immune reactions. Therefore, metabolization of ATP by ectonucleotidases CD39 and CD73 that leads to increased local amounts of adenosine prevents such a fatal development especially through triggering the adenosine receptor A2A (A2AR) on Treg and Teff cells and DC as well. The A2AR-mediated elevation of intracellular cAMP inhibits the activation of Teff cells and impairs the accessory function of DC and simultaneously strongly improves the suppressive activity of Treg cells.
