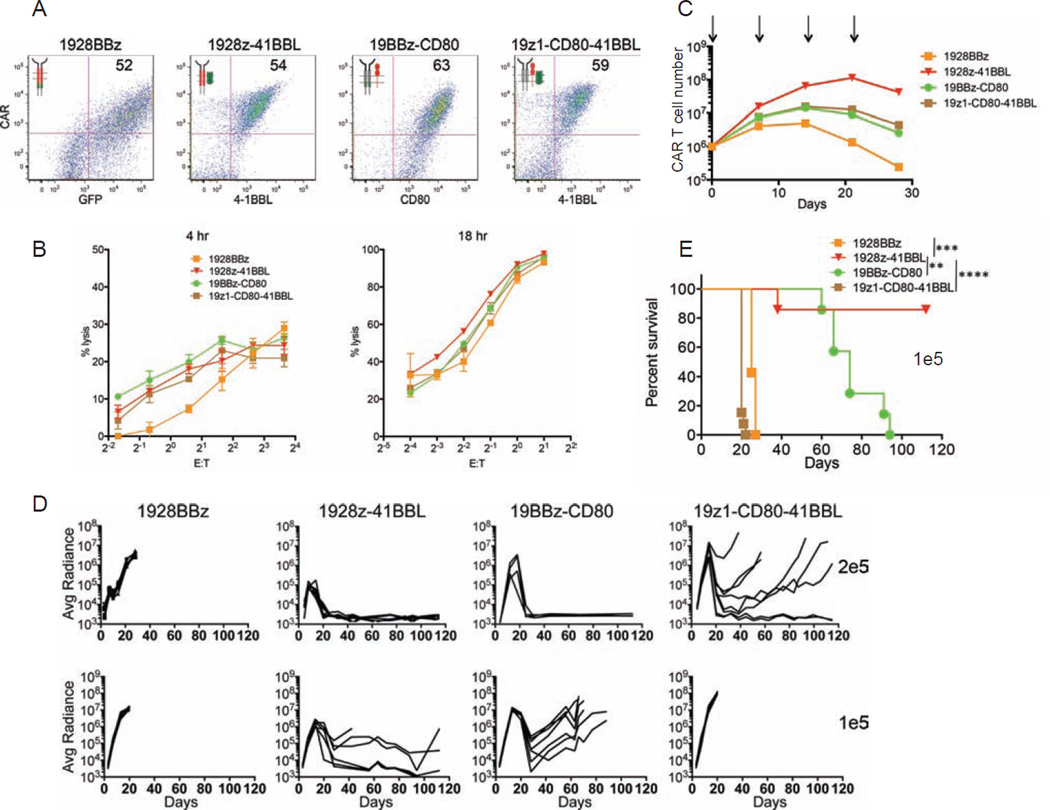
Figure 3. Therapeutic potency of a third generation CAR and three alternative combinations of costimulatory CAR designs.
(A) Flow cytometric analysis showing expression levels of the indicated CARs. (B) Cytotoxic activity using a 4 hr 51Cr release assay (left) and 18 hr bioluminescence assay (right), utilizing NALM6 cell line as targets cells. Data are means ± SD. (C) Cumulative CAR T cell counts of indicated CAR T cells upon weekly CD19 stimulation, without exogenous cytokines. Arrows indicate stimulation time points. Data are means ± SD. (D) NALM6 bearing mice were treated with 2×105 (top), or 1×105 (bottom) indicated CAR T cells. Tumor burden showed as the bioluminescent signal quantified per animal every week over a 120-day period. Quantification is the average photon count of ventral and dorsal acquisitions per animal at all given time points. Each line represents one mouse. Some groups are pooled from at least two experiments, representing n=7–14 mice per group. (E) Kaplan-Meier analysis of survival of mice in (D). *p<0.05; **p<0.01; ***p<0.001. See also Figure S3.