Abstract
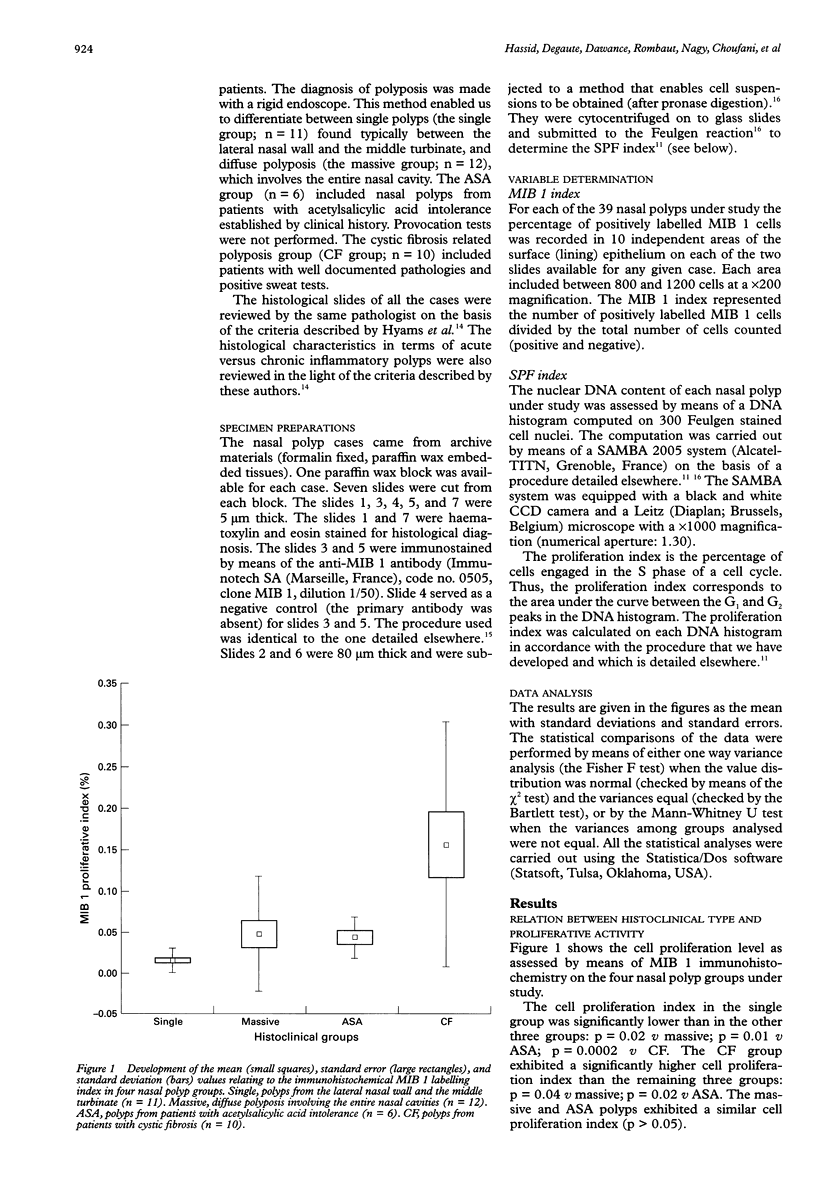
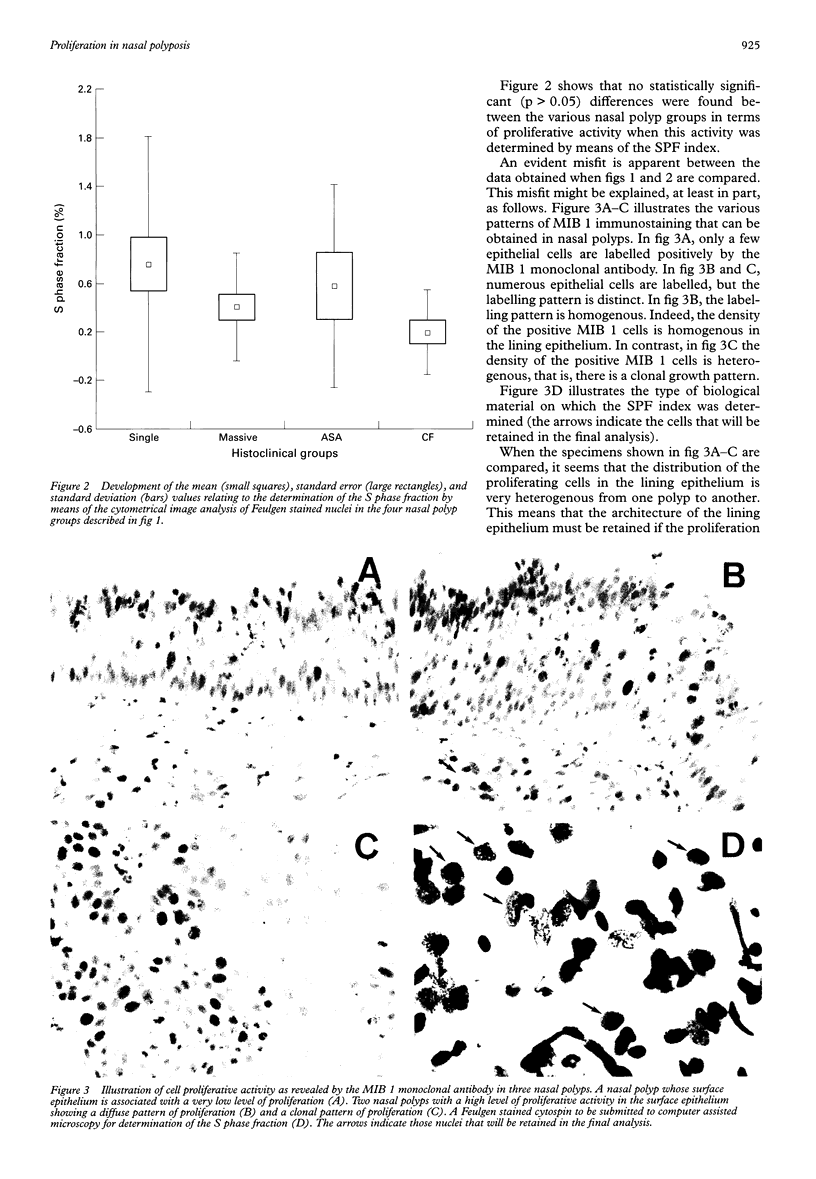
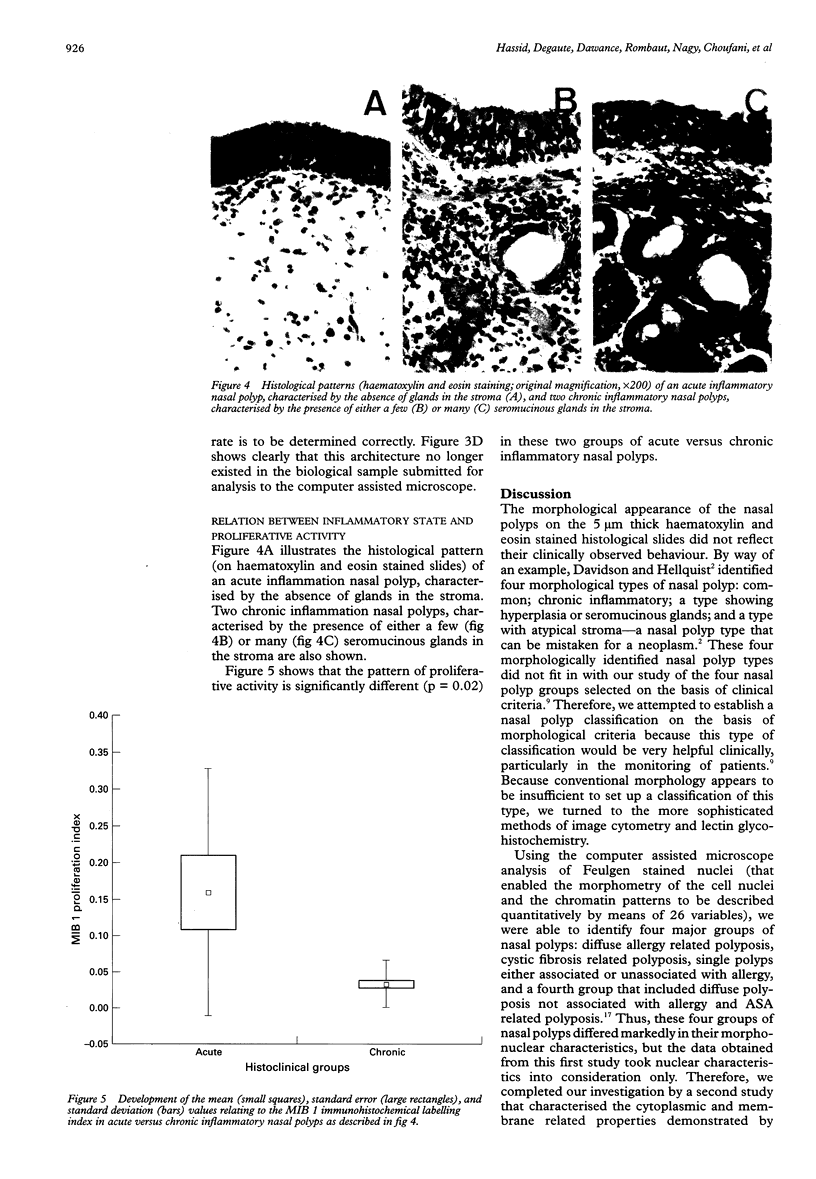
AIMS: To determine the level of proliferative activity in 39 nasal polyps with clear cut distinct clinical behaviour patterns. METHODS: The 39 nasal polyps included 11 polyps labelled as "single" and taken from the lateral nasal wall and the middle turbinate; 12 polyps labelled as "massive" and relating to diffuse polyposis involving the entire nasal cavity; six polyps labelled as "ASA" and relating to nasal polyps from patients with acetylsalicylic acid intolerance and asthma; and 10 polyps from cystic fibrosis related polyposis. Cell proliferation was determined by two independent methods: first, the computer assisted microscope analysis of isolated Feulgen stained nuclei for the measurement of the percentage of cells in the S phase of the cell cycle; and second, the immunohistochemical evaluation of a proliferation associated protein by means of the MIB 1 monoclonal antibody. RESULTS: The cystic fibrosis related polyposis exhibited the highest proliferative activity of all the clinically identified nasal polyp groups. Acute inflammatory nasal polyps exhibited a higher cell proliferation than chronic ones. The results also show that while the immunohistochemical determination of cell proliferation by means of the MIB 1 monoclonal antibody is a valuable tool in determining cell proliferation in nasal polyps, the cytometrical image analysis of Feulgen stained nuclei is not useful for this purpose. CONCLUSION: Cell proliferation activity identifies cystic fibrosis as being distinct from the other nasal polyp groups.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batsakis J. G., El-Naggar A. K. Cystic fibrosis and the sinonasal tract. Ann Otol Rhinol Laryngol. 1996 Apr;105(4):329–330. doi: 10.1177/000348949610500418. [DOI] [PubMed] [Google Scholar]
- Bernstein J. M., Gorfien J., Noble B. Role of allergy in nasal polyposis: a review. Otolaryngol Head Neck Surg. 1995 Dec;113(6):724–732. doi: 10.1016/s0194-5998(95)70012-9. [DOI] [PubMed] [Google Scholar]
- Breeze R. G., Wheeldon E. B. The cells of the pulmonary airways. Am Rev Respir Dis. 1977 Oct;116(4):705–777. doi: 10.1164/arrd.1977.116.4.705. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Becker M. H., Key G., Duchrow M., Schlüter C., Galle J., Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992 Dec;168(4):357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- Cauna N., Manzetti G. W., Hinderer K. H., Swanson E. W. Fine structure of nasal polyps. Ann Otol Rhinol Laryngol. 1972 Feb;81(1):41–58. doi: 10.1177/000348947208100105. [DOI] [PubMed] [Google Scholar]
- Coste A., Rateau J. G., Roudot-Thoraval F., Chapelin C., Gilain L., Poron F., Peynegre R., Bernaudin J. F., Escudier E. Increased epithelial cell proliferation in nasal polyps. Arch Otolaryngol Head Neck Surg. 1996 Apr;122(4):432–436. doi: 10.1001/archotol.1996.01890160072013. [DOI] [PubMed] [Google Scholar]
- Davidsson A., Hellquist H. B. The so-called 'allergic' nasal polyp. ORL J Otorhinolaryngol Relat Spec. 1993;55(1):30–35. doi: 10.1159/000276349. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Holopainen E., Mäkinen J., Paavolainen M., Palva T., Salo O. P. Nasal polyposis. Relationships to allergy and acetylsalicyclic acid intolerance. Acta Otolaryngol. 1979 Mar-Apr;87(3-4):330–334. doi: 10.3109/00016487909126428. [DOI] [PubMed] [Google Scholar]
- Kiss R., Dewitte O., Decaestecker C., Camby I., Gordower L., Delbecque K., Pasteels J. L., Brotchi J., Salmon I. The combined determination of proliferative activity and cell density in the prognosis of adult patients with supratentorial high-grade astrocytic tumors. Am J Clin Pathol. 1997 Mar;107(3):321–331. doi: 10.1093/ajcp/107.3.321. [DOI] [PubMed] [Google Scholar]
- Kiss R., Salmon I., Camby I., Gras S., Pasteels J. L. Characterization of factors in routine laboratory protocols that significantly influence the Feulgen reaction. J Histochem Cytochem. 1993 Jun;41(6):935–945. doi: 10.1177/41.6.8315284. [DOI] [PubMed] [Google Scholar]
- Salmon I., Kiss R. Relationship between proliferative activity and ploidy level in a series of 530 human brain tumors, including astrocytomas, meningiomas, schwannomas, and metastases. Hum Pathol. 1993 Mar;24(3):329–335. doi: 10.1016/0046-8177(93)90045-i. [DOI] [PubMed] [Google Scholar]
- Small P., Frenkiel S., Black M. Multifactorial etiology of nasal polyps. Ann Allergy. 1981 Jun;46(6):317–320. [PubMed] [Google Scholar]