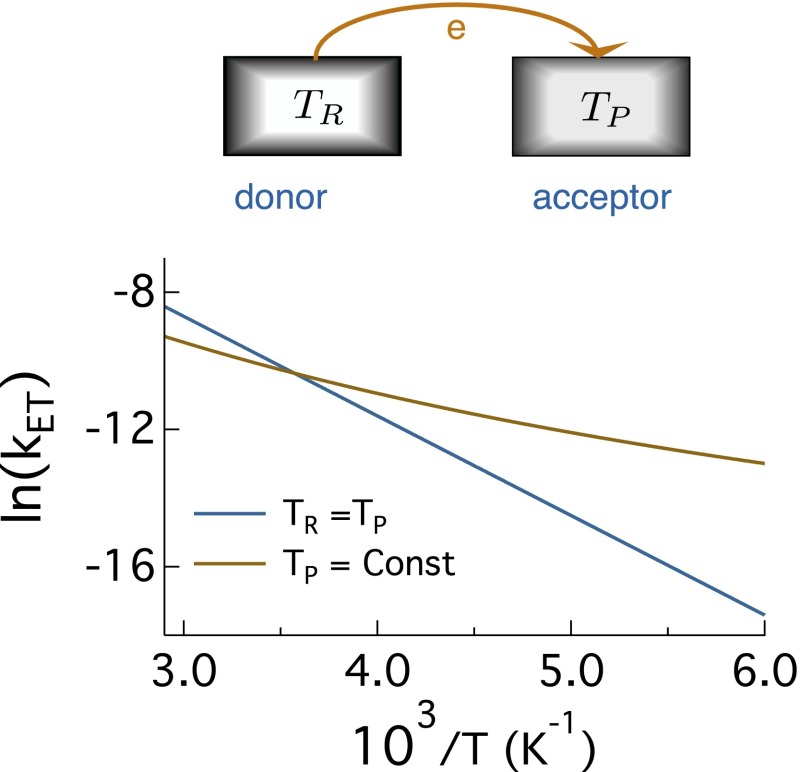
Fig. 1.
(Upper) Electron transfer between the donor and acceptor held at temperatures and , respectively. (Lower) Arrhenius plot of the logarithm of the rate constant of electron transfer vs. for the equilibrium thermal bath with and for a nonequilibrium thermal bath, in which is varied and K is held fixed. The reaction is self-exchange electron transfer with zero reaction free energy and eV; is assumed for simplicity (the rate preexponential factor is put equal to unity).