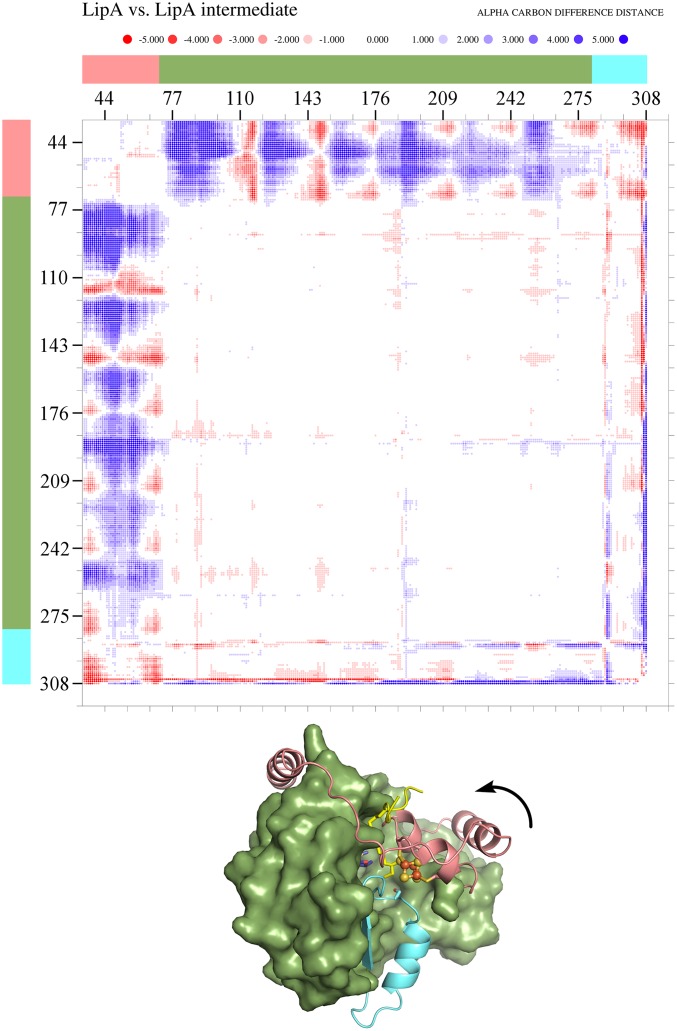
Fig. S3.
Conformational change in the N-terminal extension of LipA on substrate binding and formation of the covalent intermediate. An α-carbon difference distance matrix created using DDMP (Center for Structural Biology, Yale) shows changes in interresidue distances between the substrate-free and intermediate-bound structures. Pairs of residues that are closer to each other in the LipA intermediate structure are blue dots, whereas those that are closer in the substrate-free structure are red. The N-terminal extension (residues 4–70) is indicated in pink, the AdoMet radical core (residues 71–281) is in green, and the C-terminal extension (residues 282–311) is in cyan. Only the N-terminal extension moves substantially, with the C-terminal extension moving slightly as a result of crystal packing differences. The arrow indicates the direction of N-terminal extension movement on substrate binding and intermediate formation.