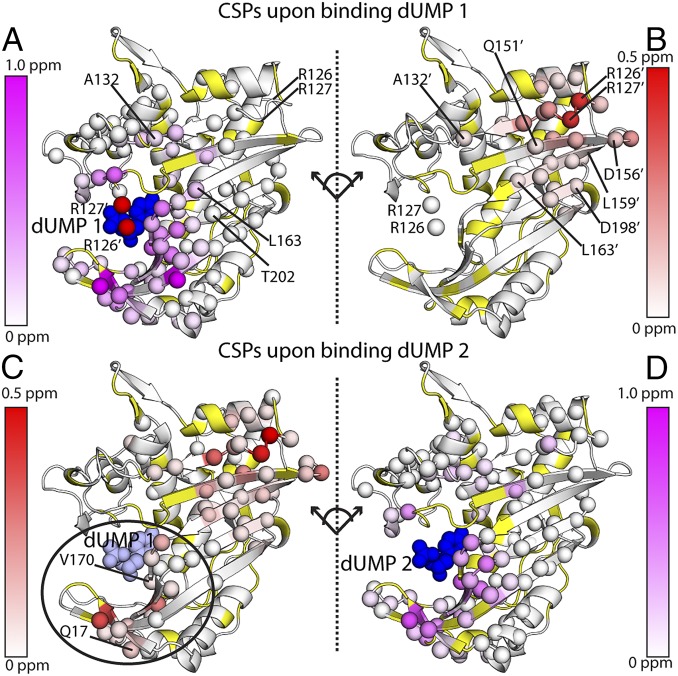
Fig. 2.
Chemical shift perturbations of the two dUMP binding events. The effects of binding the first dUMP to the binding (A) and nonbinding (B) subunits of the dimer are shown at the Top, using the CSP scheme shown in Fig. 3A. The effects of binding the second dUMP to the nonbinding (C) and binding (D) subunits are shown at the Bottom, using the reconstructed WT CSPs shown in Fig. 3B. Viewing the dimer interface region is enhanced by separating the two subunits, where the subunits on the right underwent a hinge-type rotation (dotted line) to yield the same viewing angle as those on the left. As a reference point for the rotation, the red (A) and white (B) spheres show the locations of R126 and R127 from the other subunit. Residues with significant CSPs are shown as spheres. Residues that are missing or unassigned are in yellow. Annotated residues are discussed in Figs. 4 and 5, where residues denoted prime (e.g., R126′) correspond to the empty subunit of the dUMP1 state. The first bound (dUMP 1) and second bound (dUMP 2) dUMPs are shown in dark blue; for binding of dUMP 2 (Bottom), the previously bound dUMP 1 is shown in light blue. In C, the circle highlights the major difference in the distant subunit response for binding of dUMP 1 and dUMP 2. The CSPs in context of the full dimer are shown in Fig. S1.