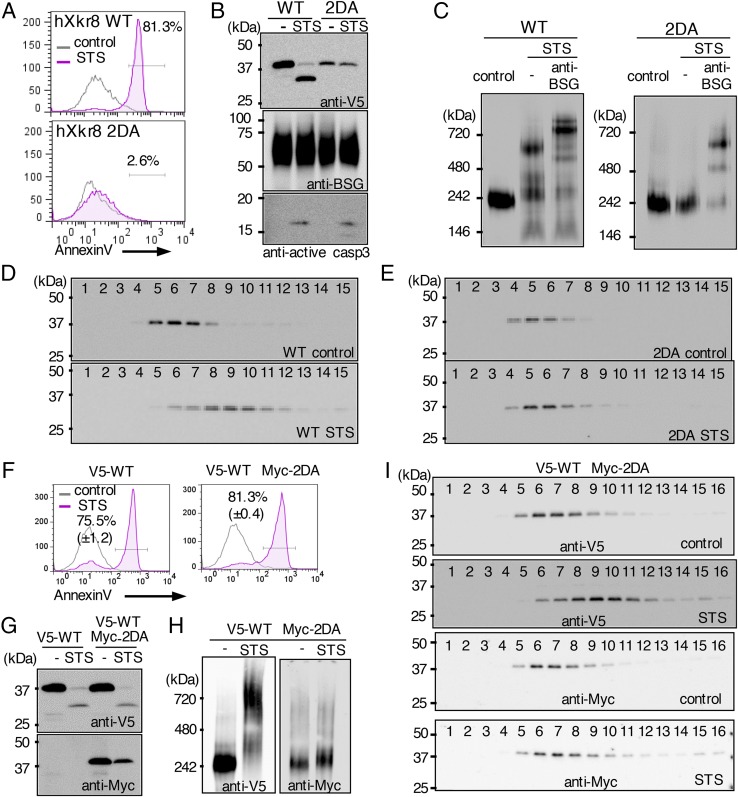
Fig. 5.
Caspase-mediated oligomerization of the Xkr8 complex. (A–C) PLB were transformed with N-terminally V5-tagged WT or 2DA Xkr8, treated or not treated with STS, stained with Annexin V, and assessed by flow cytometry (A). Cell lysates were separated by SDS/PAGE and Western-blotted with anti-V5, anti-hBSG, or anti-active caspase 3 (B). In C, crude membranes were solubilized with LMNG, separated by BN-PAGE, and Western-blotted with anti-V5. Aliquots of lysates from the STS-treated cells were incubated 1 h with anti-hBSG on ice and applied to BN-PAGE. (D and E) The LMNG-solubilized crude membranes from STS-treated or untreated PLB expressing WT (D) or 2DA hXkr8 (E) were subjected to glycerol density gradient centrifugation. Fractions were analyzed by SDS/PAGE and Western-blotted with anti-V5. (F–I) PLB transformed with V5-tagged WT alone or cotransformed with V5-tagged WT and Myc-tagged 2DA Xkr8 were untreated or treated with STS, stained with Annexin V, and assessed by flow cytometry (F). Cell lysates were separated by SDS/PAGE and Western-blotted with anti-V5 or anti-Myc (G). In H, crude membranes were solubilized with LMNG, separated by BN-PAGE, and Western-blotted with anti-V5 or anti-Myc. In I, LMNG-solubilized membranes were analyzed by glycerol density gradient centrifugation. Fractions were analyzed by SDS/PAGE and Western-blotted with anti-V5 or anti-Myc.