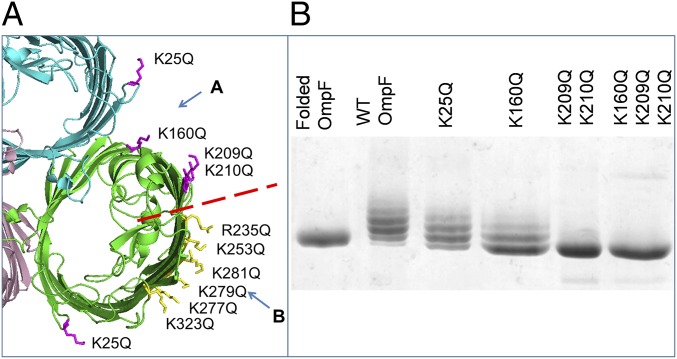
Fig. 2.
Removing positively charged residues decreases the amount of LPS bound to OmpF. (A) Localization of positively charged residues on the extracellular side of an OmpF trimer (PDB ID code 2OMF). We divided these residues into group A in the cleft (magenta) and group B at the perimeter (yellow) separated by the red dashed line. This and other structural images were created using PyMOL (65). (B) Native OmpF, WT, and site A mutant proteins, purified from the OM with bound LPS, analyzed on 10% SDS/PAGE stained with Coomassie blue. The double- and triple-glutamine mutations retain a tail of LPS-bound forms, which contrasts with the quadruple-glutamate mutations (Fig. 3F).