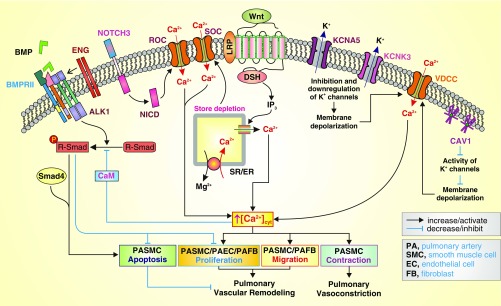
Figure 1.
Proposed mechanisms by which the genes associated with pulmonary arterial hypertension (PAH; based on emerging genetic sequencing studies) and the Wnt signaling cascade (based on the current study by Hemnes and colleagues) functionally interact with the Ca2+ signaling pathway to cause pulmonary arterial smooth muscle cell (PASMC) contraction and pulmonary vasoconstriction, PASMC proliferation/migration and pulmonary vascular wall thickening, and inhibition of PASMC apoptosis resulting in pulmonary vascular remodeling (19). On the basis of case–control studies, the current work by Hemnes and colleagues has found Wnt pathway and smooth muscle contraction pathway variants to be significantly overrepresented in PAH and in vasodilator-responsive PAH, respectively (19). Activation of Wnt signaling has been shown to induce Ca2+ release from the sarcoplasmic (SR) or endoplasmic (ER) reticulum by inositol 1,4,5-trisphosphate (IP3)-mediated activation of IP3 receptor, a Ca2+ release channel expressed in the ER/SR membrane. The Wnt-mediated Ca2+ mobilization from the SR/ER to the cytosol causes Ca2+ store depletion that subsequently opens the store-operated Ca2+ channels (SOC), formed by Orai1/2 and TRPC6 channel subunits, to induce store-operated Ca2+ entry (SOCE). Mutations in genes encoding KCNK3, a two-pore domain K+ channel, and KCNA5, a voltage-gated K+ channel, may lead to transcriptional down-regulation and/or functional inhibition of KCNK3 and KCNA5, causing membrane depolarization that opens voltage-dependent Ca2+ channels (VDCC) and induces voltage-dependent Ca2+ entry. Furthermore, up-regulated Notch3 and enhanced Notch signaling have been demonstrated to functionally activate receptor-operated Ca2+ channels (ROC) and induce receptor-operated Ca2+ entry (ROCE) via Notch intracellular domain (NICD) or other unknown mechanisms (22). Caveolin-1 (CAV1) has been shown to inhibit various K+ channels, cause membrane depolarization, and induce Ca2+ influx through VDCC. The increased cytosolic Ca2+ concentration ([Ca2+]cyt) causes PASMC contraction, leading to pulmonary vasoconstriction, and stimulates PASMC migration and proliferation, leading to pulmonary vascular remodeling (or concentric pulmonary arterial wall thickening). Bone morphogenetic protein (BMP)/bone morphogenetic protein receptor type 2 (BMPR2)/Smad signaling has been demonstrated to induce PASMC apoptosis and inhibit PASMC and pulmonary arterial endothelial cell (PAEC) proliferation (23, 24). Mutations in the BMPR2 gene identified in patients with idiopathic and heritable PAH result in the inhibition of PASMC apoptosis and enhancement of PASMC/PAEC proliferation, contributing to the development and progression of pulmonary vascular remodeling. Calmodulin (CaM) is a Ca2+-sensitive protein that physically interacts with Smad and induces Ca2+-dependent inhibition of transforming growth factor β/activin signaling (25, 26); this suggests that elevated [Ca2+]cyt and activated CaM can lead to inhibition of BMP/BMPR2/Smad signaling, and ultimately to inhibited PASMC apoptosis and enhanced PASMC proliferation. DSH = dishevelled; ENG = endoglin; LRP = low-density lipoprotein receptor–related protein; Smad = mothers against decapentaplegic homologs.