Table 1.
Physicochemical constants of (1-(4-aryl)-1H-1,2,3-triazol-4-yl)methyl, substituted phenyl-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylates 7a-l
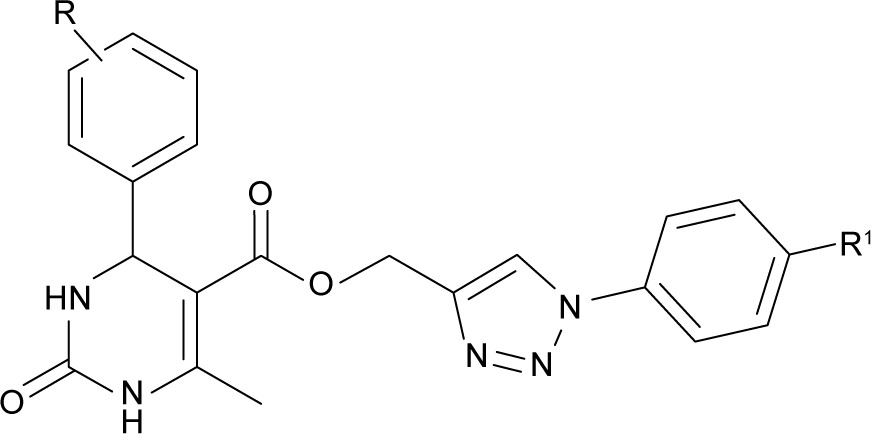
| ||||||
|---|---|---|---|---|---|---|
| Compound code | Molecular formula (molecular mass) | R | R1 | Yield (%)a,b | mp (°C) | cLogPc |
| 7a | C22H17F4N5O3 (475) | 4-F | CF | 85 | 194–196 | 4.6456 |
| 7b | C22H17F4N5O3 (475) | 3-F | CF | 87 | 178–180 | 4.6456 |
| 7c | C22H17ClF3N5O3 (491) | 3-Cl | CF | 82 | 204–206 | 5.2156 |
| 7d | C23H17F6N5O3 (525) | 4-CF3 | CF | 89 | 188–190 | 5.3856 |
| 7e | C22H17ClF3N5O3 (491) | 4-Cl | CF | 90 | 194–196 | 5.2156 |
| 7f | C23H20F3N5O4 (487) | 4-OCH3 | CF3 | 92 | 196–198 | 4.4216 |
| 7g | C23H20F3N5O4(487) | 3-OCH3 | CF3 | 87 | 198–200 | 4.4216 |
| 7h | C23H20F3N5O3 (471) | 4-CH3 | CF3 | 89 | 202–204 | 5.0016 |
| 7i | C24H22F3N5O4 (501) | 4-OC2H5 | CF3 | 88 | 214–216 | 4.9506 |
| 7j | C21H17ClFN5O3 (441) | 4-Cl | F | 77 | 144–146 | 4.3467 |
| 7k | C22H17F4N0O3 (475) | CF3 | F | 89 | 184–186 | 4.5167 |
| 7l | C21H17F2N5O3 (425) | 3-F | F | 86 | 164–166 | 3.7767 |
Notes:
All of the products were characterized by spectral and physical data.
Yields after purification by column chromatography method.
cLogP was calculated using ChemBioDraw Ultra 13.0 v.
