Figure 4.
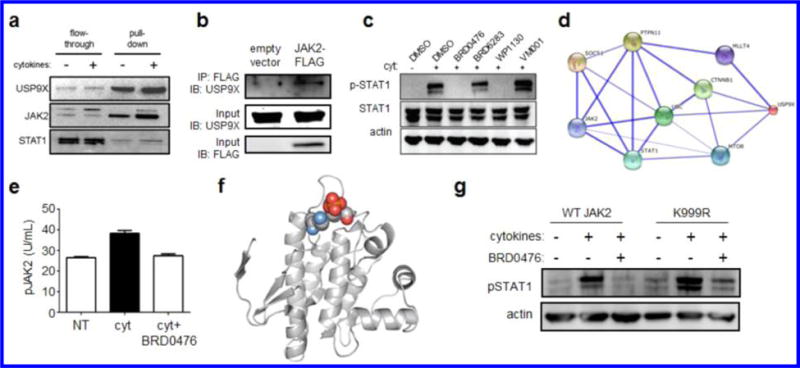
USP9X interacts with JAK2 to promote cytokine signaling, which is inhibited in a kinase-independent manner by BRD0476. (a) Affinity pull-down of USP9X and associated proteins by biotinylated BRD0476. (b) Co-immunoprecipitation of USP9X and JAK2 in INS-1E cells transfected with empty vector or JAK2-FLAG. Immunoblots of input material for total USP9X and FLAG are included. (c) Phosphorylation of STAT1 and total STAT1 protein in INS-1E cells treated 30 min with cytokines and the indicated compound. Actin was included as a loading control. (d) Network graph indicating proteins connecting JAK2 to USP9X. Weight of edges is correlated with confidence level. (e) Phosphorylation of JAK2 in INS-1E cells treated with the cytokine cocktail and 10 μM BRD0476. Data represent the mean ± standard deviation. * p < 0.0001 versus control treatment, # p < 0.001 versus cytokine treatment, Student’s t test. (f) Structural analysis of JAK2 (pdb: 4GL9),41 with the proximity of Lys999 (blue) and Tyr1007 (red) indicated. (g) Phosphorylation of STAT1 in INS-1E cells transfected with wild-type JAK2 or K999R, followed by 30 min treatment with the cytokine cocktail and 10 μM BRD0476.
