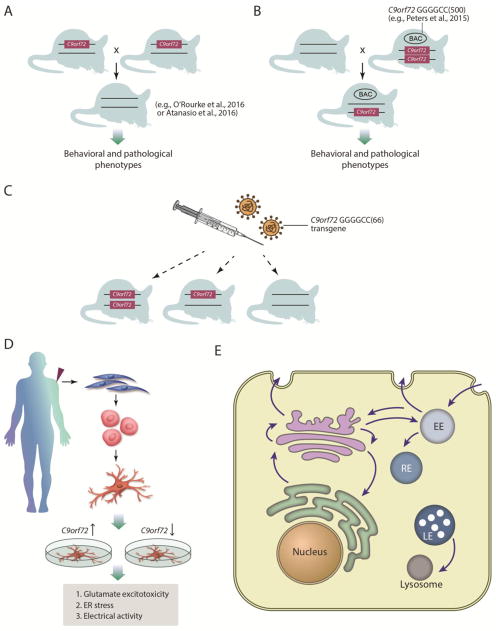
Figure 2. Additional experiments to test C9orf72 loss of function.
A) Mice have been generated in which the β–galactosidase gene replaces exons 2–6 of one of the C9orf72 alleles (Suzuki et al., 2013). These mice could be intercrossed to generate homozygous mutant mice (Atanasio et al., 2016; O’Rourke et al., 2016) and, together with their heterozygous littermates, extensively analyzed for any effects on pathological phenotypes, survival and cognitive or motor behavioral impairments. B) Crossing transgenic mice containing a human BAC with a fragment of the C9orf72 locus harboring ~500 GGGGCC repeats (e.g., Peters et al., 2015) to the C9orf72 knockout mice will test if disease is accelerated by reducing wild type C9orf72 function. C) Injecting the C9orf72 transgene (Chew et al., 2015) into the central nervous system of C9orf72 WT, +/−, or −/− animals will test if disease features are accelerated by the reduction of wild type C9orf72. D) iPS derived from c9FTD/ALS patients have been reported to exhibit phenotypic differences from control neurons, including glutamate excitotoxicity, sensitivity to ER stress, and alterations in electrical activity. If these phenotypes are due to loss of C9orf72 function, then increasing C9orf72 levels should mitigate them and lowering C9orf72 levels should worsen them. E) C9orf72 may function as a guanine nucleotide exchange factor (GEF) to regulate Rab GTPase activity. Rabs orchestrate multiple steps of membrane trafficking within cells and it will be important to define which Rab and thus which trafficking step C9orf72 regulates.