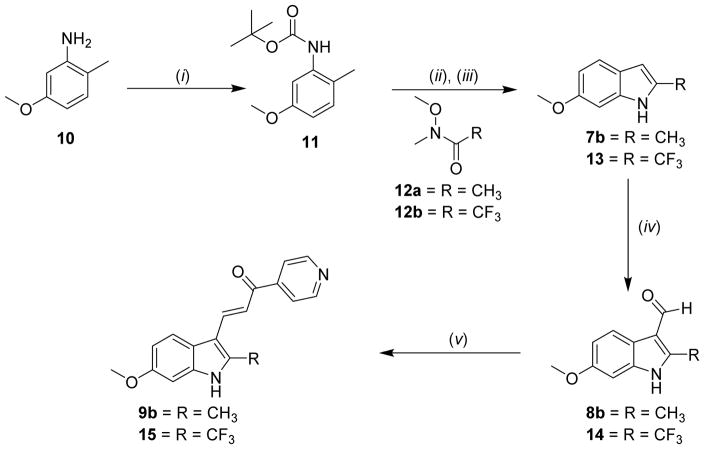
Scheme 2.
Synthesis of trifluoromethyl compound 15 and a more efficient synthesis of 9b. Reagents and conditions: (i) Boc2O, THF, reflux; (ii) THF, sec-butyllithium, −40 °C to 0 °C; then Weinreb amide (12a or 12b)/THF, −40 °C to rt; (iii) DCM/TFA; (iv) POCl3, DMF, 0 °C to rt; then 1N NaOH; (v) 4-Acetylpyridine, MeOH, reflux.