Abstract
Background
Vaginal delivery and aging are key risk factors for pelvic floor muscle dysfunction, which is a critical component of pelvic floor disorders. However, alterations in the PFM intrinsic structure due to childbirth and aging that lead to muscle dysfunction remain elusive.
Objectives
To determine the impact of vaginal deliveries and aging on human cadaveric PFM architecture, the strongest predictor of active muscle function.
Study Design
Coccygeus, iliococcygeus and pubovisceralis were obtained from younger, ≤ 51 years, vaginally nulliparous (N=5) and vaginally parous (N=6), and older, >51 years, vaginally nulliparous (N=6) and vaginally parous (N=6) donors without history of PFDs. Architectural parameters, predictive of muscle’s excursion and force-generating capacity, were determined using validated methods. Intramuscular collagen content was quantified by hydroxyproline assay. Main effects of parity and aging and the interactions were determined using two-way ANOVA, with Tukey’s post-hoc testing with significance level of 0.05.
Results
The mean age of younger and older donors differed by ~40 years (P=0.001), but was similar between nulliparous and parous donors within each age group (P>0.9). Median parity was 2 (range 1–3) in younger and older vaginally parous groups, P=0.7. The main impact of parity was increased fiber length in the more proximal coccygeus (P=0.03), and iliococcygeus (P=0.04). Aging changes manifested as decreased physiological cross sectional area across all pelvic floor muscles, P<0.05, which substantially exceeded the age-related decline in muscle mass. Physiological cross sectional area was lower in younger vaginally parous, compared to younger vaginally nulliparous pelvic floor muscles, however the differences did not reach statistical significance. Pelvic floor muscles’ collagen content was not altered by parity, but increased dramatically with aging, P<0.05.
Conclusions
Increased fiber length in more proximal pelvic floor muscles likely represents an adaptive response to the chronically increased load placed on these muscles by the displaced apical structures, presumably as a consequence of vaginal delivery. In younger specimens, a consistent trend towards decrease in force generating capacity of all pelvic floor muscles in parous group suggests a potential mechanism for clinically identified pelvic floor muscle weakness in vaginally parous women. The substantial decrease in predicted muscle force production and fibrosis with aging represent likely mechanisms for the pelvic floor muscle dysfunction in older women.
Keywords: pelvic floor muscles, architecture, vaginal delivery, aging
Introduction
Pelvic floor disorders (PFDs) include pelvic organ prolapse, urinary, and fecal incontinence. Collectively, they represent a major public health problem given their high prevalence, negative impact on quality of life, lack of preventive measures, high failure rate of available treatments, and associated economic burden (1–3). The etiology of these complex disorders is not well understood but thought to be multifactorial, with vaginal delivery and aging identified as the major inciting and promoting events (4–6). These risk factors are thought to destabilize structural components integral to the proper function of female pelvic floor, including pelvic floor skeletal muscles (PFMs).
Pelvic floor muscles are comprised of the levator ani complex and coccygeus, which make up the dorsal part of the pelvic floor and is intimately associated with the posterior portion of the levator ani. PFMs counteract gravitational force and resist intra-abdominal pressure, providing support to pelvic and abdominal viscera and aid in urinary and fecal continence. Despite this, current knowledge pertaining to structure, function, and pathology of human PFMs is severely limited because directly probing these muscles in vivo is not feasible due to their location deep in the pelvis. The majority of research focused on PFMs relies on imaging, computational modeling, or indirect clinical assessment. Although such investigations have been invaluable in establishing a framework for the role of PFM dysfunction in the pathogenesis of PFDs (7, 8), significant knowledge gaps remain. Importantly, decreased PFM strength precedes the onset of symptomatic PFDs (9, 10); a third of women with pelvic organ prolapse do not exhibit radiological defects in PFMs (8) and stress urinary incontinence is more frequent in women with minimal, compared to major, levator ani defects on magnetic resonance imaging (MRI) (11). Prolapse and urinary incontinence can, of course, result from a compromise in the integrity of the striated urethral sphincter or pelvic connective tissue supportive structures. Alternatively, alterations in the intrinsic PFM structure, not detectable by traditional imaging modalities, may impair muscle function in the absence of radiologically evident defects.
Skeletal muscle architecture governs the magnitude of force a muscle generates, how fast it contracts (velocity), and its range of contraction (excursion) (12). A shift in architectural design directly impacts functional capacity. Therefore, measuring alterations in architecture provides insight into the mechanics of PFM dysfunction and allows to overcome the limitations inherent to the radiologic investigations, which invoke an anatomical cross-sectional area that has been previously described to be a poor proxy measure for actual muscle force generating capacity (12). The above is especially pertinent to the examination of aging effects on skeletal muscles, as loss of muscle strength with age exceeds that of muscle mass (13, 14). Marked alterations in muscle architecture caused by aging, resulting in loss of sarcomeres both in parallel and in series, partially explain this finding (14–16). Furthermore, radiologic studies have failed to identify age-related atrophy as an essential component of PFM dysfunction in older women (17). We believe that standard imaging techniques are limited due to their inability to distinguish among and identify changes in the contractile and intramuscular extracellular (ECM) components of PFM, which are remarkably thin, compared to other human skeletal muscles (18). Studies probing muscle architecture are highly invasive and not feasible in living women, thus human cadaveric specimens serve as an invaluable source of tissue (19) and a number of transformative concepts in the field of musculoskeletal medicine resulted from investigations of cadaveric limb muscles (20–22).
The goal of this study was to determine the independent and combined effects of vaginal childbirth and aging, the leading epidemiological risk factors for PFM dysfunction, on muscle architecture in the absence of significant PFD by directly examining human cadaveric PFM specimens. We tested the following hypotheses: 1) because of their distinct proximal-distal spatial distribution, the architecture of individual PFMs is variably but permanently altered by vaginal delivery; 2) similar to other human skeletal muscles, age-related changes in the PFM architectural parameters deleteriously impact muscle predicted force production.
Materials and Methods
Performing this study required unique access to well-defined cadaveric specimens in which age and parity status, as well as history of symptomatic PFDs could be unambiguously determined. Thus, we partnered with the University of Minnesota Bequest Body Donation Program, which provides access to donors’ medical records. The study was exempt from institutional review board approval due to exclusion of living human subjects. Specimens were obtained from donors without history of PFDs or rectal prolapse, to eliminate potential confounding effects of disease on architecture. Donors with history of pelvic radiation, gynecologic or colorectal malignancy, pelvic metastasis, connective tissue disorder, myopathy, colectomy or proctectomy were also excluded. In contrast to vaginal delivery, PFM injury is rarely observed after cesarean childbirth (23, 24), and PFM strength is unchanged by abdominal deliveries (9). Thus, nulliparous donors (n=8) and donors with a history of Cesarean deliveries only (n=3) were designated as vaginally nulliparous. Over a 2 and a half year period, we accumulated 23 specimens which enabled statistical comparison among the following 4 groups: younger vaginally nulliparous (YVN, n=5), younger vaginally parous (YVP, n=6), older vaginally nulliparous (OVN, n=6), and older vaginally parous (OVP, n=6). Based on epidemiologic studies, age surpasses parity as a risk factor for PFDs after menopause (25), which is a marker of biological aging in women (26). Using statistics from the National Institute of Aging, the average age of menopause in the U.S. is 51. Thus, we defined “younger” as ≤ 51 and “older” group as > 51 years of age, which is consistent with previously reported onset of age-related sarcopenia in the 6th decade of life (27, 28).
Muscle Architecture
To precisely maintain architectural structure, coccygeus (C), iliococcygeus (IC), and pubovisceralis (PV), consisting of pubococcygeus and puborectalis, were perfusion fixed in situ (i.e., attached to the skeleton) via injection of formaldehyde through the common carotid artery, in contrast to most studies that immersion fix tissues. PFMs were harvested en block and separated into individual muscles, identified by tracking each muscle along its length, weighed and divided into cephalad, middle, and caudate regions, as previously described (18). Muscle thickness was measured with electronic calipers to the nearest 0.01 mm in each region. The following architectural parameters were determined using validated methods (29): muscle mass; physiological cross sectional area (PCSA), a predictor of isometric force generation capacity; fiber length (Lf), a predictor of muscle excursion and contractile velocity; and sarcomere length (Ls), which determines force produced when muscle is stimulated. Three fiber bundles were dissected from each region and Lf was measured with electronic calipers to the nearest 0.01 mm. Myofibers were isolated from each bundle under a dissecting microscope using a 6X objective and an overall magnification of 60X (Leica MZ16, Meyer Instruments Inc., Houston, TX) and mounted on a slide for Ls determination by laser diffraction (30). The fixation process shortens Ls by ~10% of its in vivo length, thus Ls provides information regarding muscle’s in vivo sarcomere length. Ls is also used to calculate the number of sarcomeres in series within fibers to normalize fiber length (Lfn) and correct for potential differences in length among specimens at the time of fixation. Physiological cross-sectional area was calculated as previously described, using an optimal human sarcomere length of 2.7µm (31).
Intramuscular ECM
PFMs have a composite structure consisting of the contractile myofibers embedded within a large connective tissue network of ECM, which primarily consists of collagen (32). Muscle ECM determines muscle passive mechanical properties and muscle ability to sustain load (32–34). Pathological accumulation of intramuscular collagen or fibrosis negatively impacts muscle mechanical properties and may account for the insensitivity to rehabilitation of limb skeletal muscles (35). Elucidating the impact of vaginal delivery and aging on the PFMs intramuscular ECM is important to advance our understanding of potential changes in muscle properties and lead to novel therapies. Hydroxyproline quantification was thus used to define intramuscular collagen content using a modification of a validated protocol (36, 37). Total collagen content was determined by converting hydroxyproline concentration using the constant of 7.46, the number of hydroxyproline residues per collagen molecule.
Statistical analyses
A fully crossed experimental design was used to assess independently the effects of parity and aging on individual PFMs. The main effects of parity and aging and their interaction were determined by two-way and repeated measures analysis of variance (ANOVA), followed by multiple comparisons with Tukey’s range test, as appropriate. The important interaction term addressed the following question: Does vaginal parity alter the effects of aging on PFM structural properties? Demographic variables were compared among groups by one-way ANOVA and Student t-test. Significance level (α) was set to 5%. To obtain 80% power (1-β) to detect a 20% difference the sample size of 5/group was calculated utilizing the equation: n=(CV)2/(ln(1-δ))2 using the experimental variability from YVN group with variables n=sample size; δ=difference desired (15%); CV=coefficient of variation (38, 39). All data were screened for normality and skew in order to satisfy the assumptions of the parametric tests used. Results are presented as mean ± standard error of the mean (SEM), except where noted. All statistical analyses were performed using GraphPad Prism version 6.00, GraphPad Software, San Diego, CA, USA.
Results
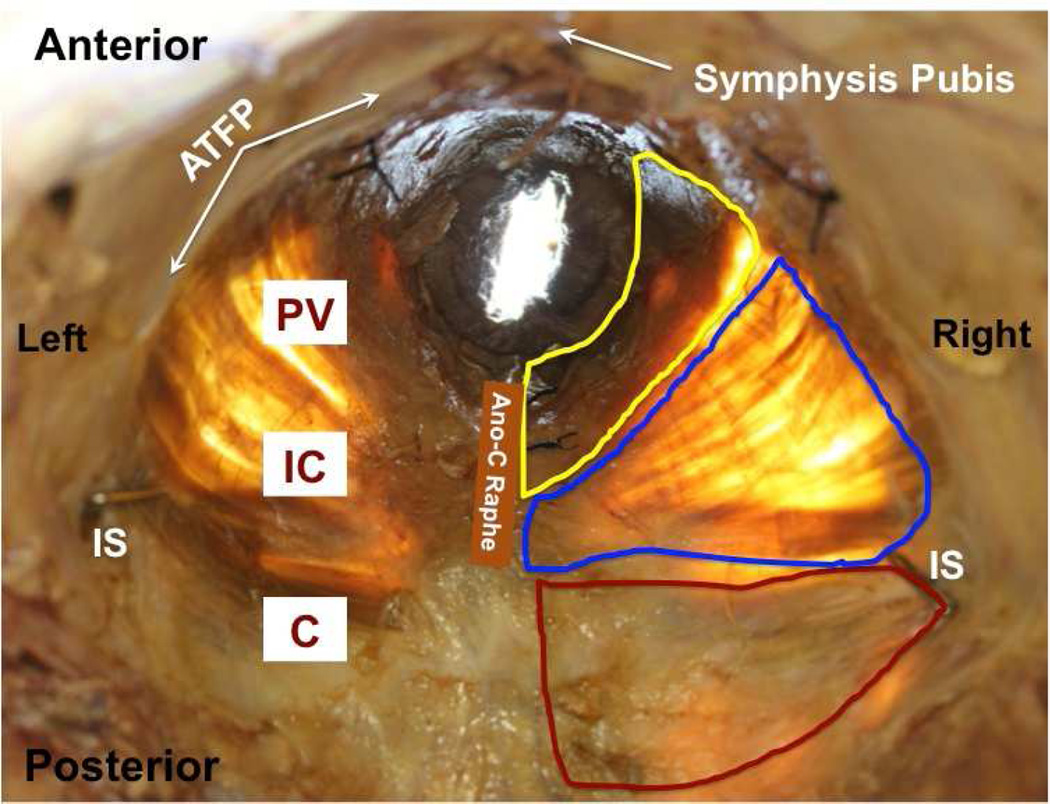
The difference in the mean age of donors between younger and older groups was highly significant and nearly 40 years (Table 1) permitting a meaningful quantification of an age effect. Within study groups, the age of nulliparous and parous donors did not differ (Table 1) ensuring that ages of subgroups did not confound the experimental design and obviating the need for analysis of covariance (ANCOVA). Also importantly, body mass index was similar for all groups, eliminating another potential confound (Table 1). Younger and older parous donors did not differ with respect to parity (Table 1). During dissection, gross disruption at entheses, lateral attachments to the arcus tendinious levator ani, or within muscle bellies did not occur for any of the 23 specimens. There were no differences between the right and left sides or the three regions of the individual muscles. All PFMs were extremely thin, as previously reported (18), nearly transparent, irrespective of age or parity, with similar muscle thickness among groups (Fig. 1). Among individual PFMs, thickness of C was 3.1±0.2 mm, which was significantly greater than 1.7±0.1 mm and 1.9±0.1 mm thickness of IC and PV, respectively (P<0.001).
Table 1.
Demographic variables of cadaveric donors
| Group | Age (years) Mean (SEM) |
BMI (kg/m2) Mean (SEM) |
Parity Median (range) |
|---|---|---|---|
| Younger VN (N=5) | 40.4 (5.4) | 21.1 (2.6) | |
| Younger VP (N=6) | 43.5 (2.4) | 20.8 (1.8) | 2 (1–3) |
| Older VN (N=6) | 80.0 (6.7) | 19.2 (1.6) | |
| Older VP (N=6) | 81.0 (7.7) | 17.8 (1.1) | 2 (1–4) |
| P-value* | P-value^ | ||
| YVN vs. YVP | 0.98 | 0.99 | |
| YVN vs. OVN | 0.001 | 0.89 | |
| YVP vs. OVP | 0.001 | 0.62 | 0.76 |
| OVN vs. OVP | 0.99 | 0.94 | |
P values derived from Tukey’s pairwise comparisons, following two-way analysis of variance
P values derived from Student t-test, with significance level set to 5%. VN: vaginally nulliparous; VP: vaginally parous. YVN: younger vaginally nulliparous; YVP: younger vaginally nulliparous; OVN: older vaginally nulliparous; OVP: older vaginally parous.
Figure 1.
Representative photograph of in situ pelvic floor muscles from a younger donor (superior view) with superimposed boundaries of coccygeus (C, red), iliococcygeus (IC, blue), and pubovisceralis (PV, yellow).
ATFP: arcus tendinious fascia pelvis; IS: ischial spine; Ano-C Raphe: ano-coccygeal raphe.
Muscle Architecture
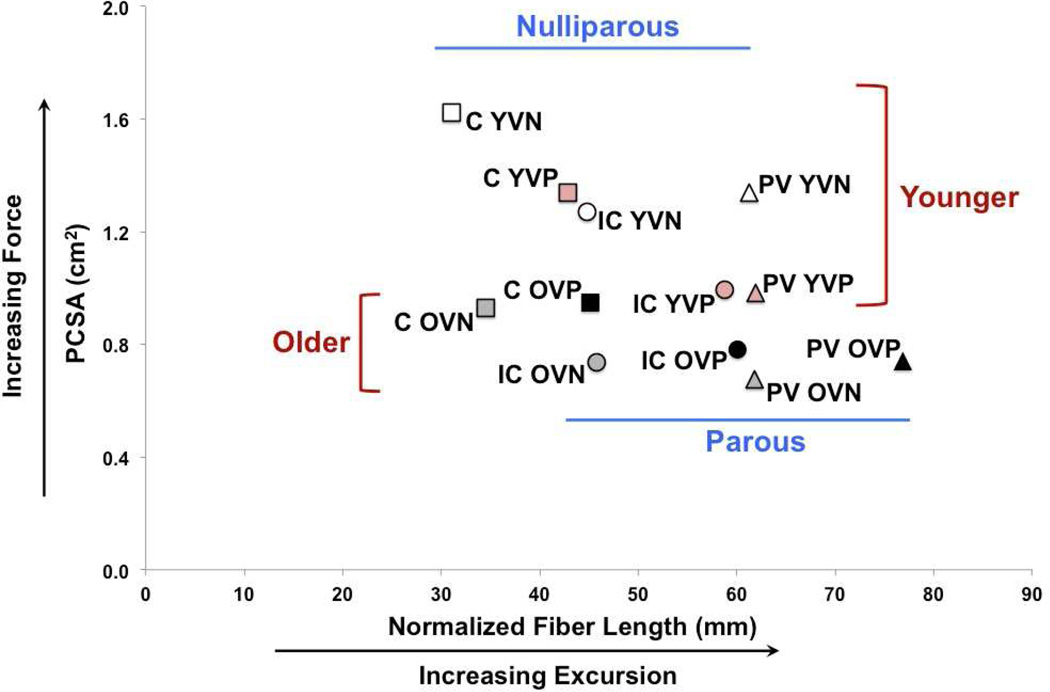
Vaginal parity and aging had significant but differential effects on PFM architecture and the magnitude of the effect varied by muscle, as illustrated by the percent of total variation and the mean squares term (Table 2). There were no significant interactions between aging and vaginal parity (Table 2). The relationship between the PCSA and normalized fiber length expresses both the force-producing and excursion capability of a muscle (12) and is displayed for the individual PFMs in each group (Fig. 2). The main impact of parity was increased fiber length in the more proximally located C and IC, while aging-related changes manifested as decreased PCSA across all PFMs (Fig. 2).
Table 2.
Summary of analysis of variance for architectural parameters of individual pelvic floor muscles, including the main effects and the interaction of vaginal parity and aging.
| Percent of Total Variation (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle | Normalized Fiber Length |
PCSA | Sarcomere Length | Muscle Mass | ||||||||
| Parity | Aging | Inter. | Parity | Aging | Inter. | Parity | Aging | Inter. | Parity | Aging | Inter. | |
| Coccygeus | ||||||||||||
| 48.9 | 3.2 | 0.2 | 2.8 | 47.8 | 3.7 | 0.1 | 26 | 7.5 | 9.7 | 34.9 | 0.05 | |
| MS | 714.3 | 46.1 | 2.2 | 0.1 | 1.7 | 0.1 | 0.0005 | 0.2 | 0.06 | 5.4 | 19.4 | 0.03 |
| P-value | 0.0003 | 0.3 | 0.8 | 0.3 | 0.0003 | 0.2 | 0.9 | 0.01 | 0.2 | 0.08 | 0.002 | 0.9 |
| Iliococcygeus | ||||||||||||
| 50.5 | 0.4 | 0.01 | 3.4 | 32.9 | 5.4 | 1.8 | 2.1 | 0.03 | 4.9 | 31.6 | 4.1 | |
| MS | 1146.0 | 8.0 | 0.1 | 0.1 | 0.8 | 0.1 | 0.03 | 0.03 | 0.0005 | 3.1 | 19.7 | 2.6 |
| P-value | 0.0003 | 0.7 | 0.9 | 0.3 | 0.005 | 0.2 | 0.6 | 0.5 | 0.9 | 0.2 | 0.005 | 0.3 |
| Pubovisceralis | ||||||||||||
| 12.4 | 11.9 | 10.5 | 3.8 | 31.7 | 6.6 | 0.1 | 1.2 | 3.3 | 0.003 | 14.9 | 11.8 | |
| MS | 349.2 | 337.7 | 296.7 | 0.1 | 1.3 | 0.3 | 0.001 | 0.01 | 0.04 | 0.003 | 15.2 | 12.0 |
| P-value | 0.07 | 0.07 | 0.09 | 0.3 | 0.005 | 0.2 | 0.9 | 0.6 | 0.4 | 0.9 | 0.07 | 0.1 |
P-values derived from F-statistics with significance level set to 5%. PCSA: physiological cross-sectional area. MS: mean squares, which measure contribution of parity, aging, and interaction terms to the variation in the PFM architectural parameters.
Figure 2.
Scatter plot of normalized fiber length and physiological cross-sectional area (PCSA) of coccygeus (C), iliococcygeus (IC), and pubovisceralis (PV) muscles.
YVN: younger vaginally nulliparous; YVP: younger vaginally nulliparous; OVN: older vaginally nulliparous; OVP: older vaginally parous
Impact of Vaginal Parity on PFMs
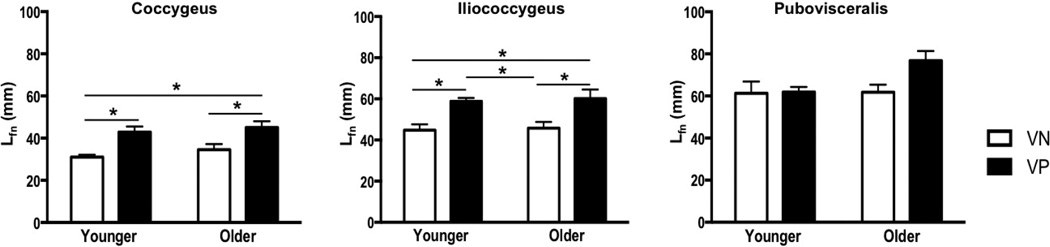
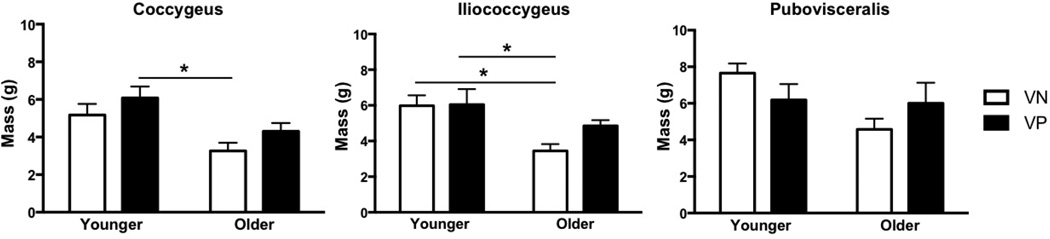
Vaginal delivery resulted in a significantly longer Lfn in C and IC in parous relative to nulliparous specimens in both younger and older groups. C Lfn increased by 38% in younger (P=0.02) and 30% in older group (P=0.04) and IC Lfn increased by 31% in younger (P=0.03) and older groups (P=0.02) (Fig. 3). However, PV Lfn did not differ between vaginally nulliparous and parous groups, irrespective of age (younger: P>0.9; older: P=0.07). PCSA was lower in all PFMs in YVP compared to YVN specimens, however the differences did not reach statistical significance (Fig. 4). In older group, vaginal delivery did not impact PCSA (Fig. 4). PFMs in YVN and YVP groups had Ls near optimal sarcomere length (Fig. 5). Muscle mass did not differ between parous and nulliparous specimens in either younger or older groups (Fig. 6).
Figure 3.
Changes in normalized fiber length (Lfn) of the pelvic floor muscles with vaginal parity and aging
*P values derived from Tukey’s pairwise comparisons, following two-way analysis of variance with significance level set to 5%. VN: vaginally nulliparous; VP: vaginally parous
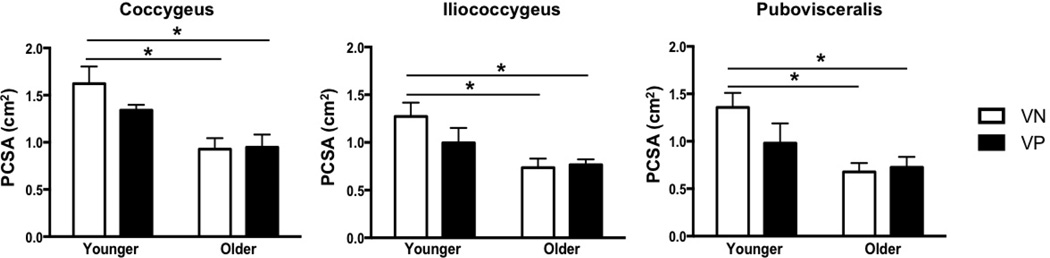
Figure 4.
Changes in physiological cross-sectional area (PCSA) of the pelvic floor muscles with vaginal parity and aging
*P values derived from Tukey’s pairwise comparisons, following two-way analysis of variance with significance level set to 5%. VN: vaginally nulliparous; VP: vaginally parous
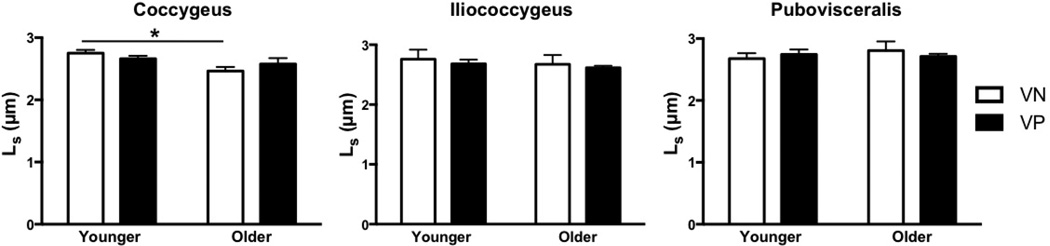
Figure 5.
Effects of vaginal parity and aging on the pelvic floor muscles’ sarcomere length (Ls)
*P values derived from Tukey’s pairwise comparisons, following two-way analysis of variance with significance level set to 5%. VN: vaginally nulliparous; VP: vaginally parous
Figure 6.
Effects of vaginal parity and aging on the pelvic floor muscles’ mass
*P values derived from Tukey’s pairwise comparisons, following two-way analysis of variance with significance level set to 5%. VN: vaginally nulliparous; VP: vaginally parous
Impact of Aging on PFMs
In contrast to vaginal delivery aging did not impact Lfn (Fig. 3). However, PCSA of all muscles was significantly lower in OVN compared to YVN group, with a dramatic decrease of 43% in C, P<0.01, 42% in IC P=0.03, and 50% in PV, P=0.02 (Fig. 4). Importantly, age-related decrease in PCSA was not significant in YVP, compared to OVN group, with a 30% decline in C, P=0.1, 26% in IC, P=0.4, and 31% in PV, P=0.5 (Fig. 3). Furthermore, PFM PCSA did not differ between YVP and OVP groups, P>0.1 (Fig. 4). Sarcomere length was significantly shorter in C in OVN, compared to YVN group: 2.46 µm vs. 2.75µm, (P=0.04). Maximum myosin-actin overlap and associated maximum active tension that muscle generates upon stimulation occurs at Ls of 2.7µm in human muscle. Thus, the shorter Ls in OVN specimens would result in lower C active force production. The extent of age-related decrease in muscle mass was substantially smaller than the changes observed in PCSA (Fig. 6).
Intramuscular ECM
Similar to the distinct impact on PFM architecture, vaginal delivery and aging had a differential main effect on PFM extracellular matrix total collagen content, with variable magnitude of the effect on the individual PFMs. Collagen content was not altered by parity in either younger or older groups (Table 3). On the other hand, aging resulted in a dramatic increase in intramuscular collagen in all PFMs of OVN compared to YVN, with 44% in C, 51% in IC, and 72% in PV (Table 3).
Table 3.
Total intramuscular extracellular matrix collagen content, expressed in micrograms of collage per milligram of tissue.
| Group | Coccygeus Mean (SEM) |
Iliococcygeus Mean (SEM) |
Pubovisceralis Mean (SEM) |
|---|---|---|---|
| Younger VN (N=5) | 81.4 (4.7) | 52.9 (4.3) | 50.3 (8.7) |
| Younger VP (N=6) | 76.0 (6.9) | 49.1 (8.5) | 69.0 (8.4) |
| Older VN (N=6) | 117.5 (7.9) | 79.8 (3.1) | 86.4 (7.5) |
| Older VP (N=6) | 121.7 (9.8) | 90.2 (5.8) | 110.6 (4.4) |
| P-value* | |||
| YVN vs. YVP | 0.98 | 0.99 | 0.45 |
| YVN vs. OVN | 0.03 | 0.03 | 0.02 |
| YVP vs. OVP | 0.01 | 0.0005 | 0.003 |
| OVN vs. OVP | 0.99 | 0.77 | 0.16 |
P values derived from Tukey’s pairwise comparisons, following two-way analysis of variance with significance level set to 5%. VN: vaginally nulliparous; VP: vaginally parous. YVN: younger vaginally nulliparous; YVP: younger vaginally nulliparous; OVN: older vaginally nulliparous; OVP: older vaginally parous.
Comment
To identify structural changes that potentially govern PFM functional alterations as a consequence of vaginal parity and aging, we examined human cadaveric PFM specimens, obtained from donors without history of PFD. These data demonstrate that parity leads to increased fiber length in more proximal PFMs. The above likely represents an adaptive response to variable functional conditions placed on those muscles, presumably as a consequence of vaginal delivery, which is clearly not reversed. Previous studies demonstrated that vaginal delivery leads to displacement of pelvic structures, primarily at the apex (40–42). Apical descent would result in chronically increased load predominantly exerted on more posterior and proximally located C and IC. In response, these muscles appear to adjust their architectural design by adding sarcomeres in series to increase their functional excursion. Increase in fiber length may also allow these muscles to maintain maximum dynamic force production despite greater mechanical strain from apical displacement. Fiber length of PV, on the other hand, was not affected by parity, likely because of its more distal location further away from vaginal apex. Longer baseline PV fiber length in nulliparous donors may also play a role. We suspect that in the absence of a greater mechanical strain placed on the most distally located PV in donors without symptomatic PFDs, the lack of fiber length change is unlikely to impact function of this muscle. We anticipate that PV fiber length would similarly increase in response to a rising mechanical load in donors with more distally displaced pelvic organ prolapse. With respect to physiological cross-sectional area, even though statistical significance was not achieved, the predicted isometric force generating capacity of all PFMs was decreased in younger vaginally parous group, compared to younger nulliparous controls. This finding suggests a potential mechanism for clinically identified PFM weakness in vaginally parous women, preceding the onset of symptomatic PFD (9, 10). We anticipate that the parity-related changes in the PFM architectural design, identified in this study, are likely more pronounced in the presence of symptomatic PFDs.
In addition to vaginal deliveries, aging is another key risk factor for PFM dysfunction. Age-related sarcopenia is an important determinant of skeletal muscle weakness, associated with untoward health consequences in older people. However, loss of muscle strength with age exceeds that of muscle mass (13, 14). Marked alterations in muscle architecture caused by aging, resulting in loss of sarcomeres both in parallel and in series, explain this phenomenon (14–16). From a functional point of view, sarcomere number reduction alters length–tension and the force–velocity relationships (12). Comparisons of maximum shortening velocity and isometric force production in limb muscles demonstrate that changes in muscle architecture account for 50% of the loss in muscle function in older individuals (16, 28). In addition, it is well established that aging leads to abnormal accumulation of collagen in the intramuscular ECM, termed fibrosis (43–45). Existing data on the effects of aging on PFMs are scarce. Clinical studies demonstrate that aging is associated with PFM weakness and impaired response to rehabilitative training (46, 47). The results of the current study show that age-related changes impact the architecture and the collagen content of the PFMs. The data support our hypothesis that aging causes a substantial decrease in the PFM predicted force production and fibrosis. One of our key findings is the lack of interaction between age and vaginal parity, highlighting that age-related detrimental alterations in intrinsic structure of the PFMs occur independent of childbirth. These results are consistent with the epidemiological data that identify aging as the strongest risk factor for the progression to symptomatic PFDs after menopause, with many older women developing PFDs independent of parity (48, 49). In the future, we will assess if significant interactions between these two major risk factors exist in the presence of PFDs. These findings improve our understanding of the mechanisms that lead to the pathological transformation of PFMs and their decreased response to rehabilitation in older women. Furthermore, the results of this investigation provide impetus for using PFM rehabilitation as a preventive strategy, aimed at mitigating the untoward impact of aging prior to the development of significant fibrotic changes. Studies in limb and trunk muscles showed that exercise may offset age-related changes. However, PFM exercises, while utilized as a treatment for women with PFDs, are currently not used preemptively, partially because aging effects on the PFMs have not been detected in older nulliparous women using standard imaging modalities (17). Lastly, characterization of age-induced changes in the intrinsic components of PFMs sets the stage for utilizing novel radiological approaches, better suited for exploring these parameters in living women.
We are aware of the limitations associated with the use of cadaveric specimens. With respect to muscle architecture, we appreciate that the PFMs were fixed resulting in a different topographical orientation from that observed in vivo. Fortunately, such differences in the topology do not affect major determinants of muscle architecture (50). To assure accurate comparisons of the architectural parameters, we normalized fiber length to sarcomere length to account for any potential differences in fiber length due to variable tilting of the pelvis at the time of death and fixation process (51). Secondly, factors other than changes in muscle architecture can also impact PFM function. Specifically, birth injury and age-related neuropathy could, of course, play an important role in functional compromise of the PFMs; however histomorphological studies indicate that alterations due to aging are consistent with myogenic origin, with no evidence of neurogenic damage in the PFMs (52, 53). The myogenic origin of age-related decline in strength is also supported by the investigations of limb muscles, in which weakness with aging was not due to the decline in neural drive (54). Finally, the external validity of the PFM architectural parameters might be limited due to the additional obstetrical factors not controlled for in the current study. However, variations in donors’ demographic characteristics are unlikely to impact the findings, as the architecture of a specific muscle is conserved between individuals of the same species (12).
Conclusion
In conclusion, our data reveal increased fiber length in more proximal PFMs and a consistent trend towards decrease in force generating capacity of all PFMs as a consequence of vaginal delivery. Secondly, aging results in substantial decrease in the predicted force production and fibrosis in all PFMs. Importantly, the lack of interaction between aging and vaginal parity highlights that age-related detrimental alterations in intrinsic structural components of the PFMs occur independent of childbirth. We are optimistic that these findings provide insight into the causes of vaginal childbirth and age-related alterations of the PFMs and serve as the foundation for future translational studies.
Acknowledgments
The authors wish to thank individuals who donated their bodies to the University of Minnesota’s Anatomy Bequest Program for the advancement of education and research.
This research was presented at the combined American Urogynecologic Society/International Urogynecological Association’s annual scientific meeting in July 2014, Washington DC and at the American Urogynecologic Society’s annual scientific meeting in October 2015, Seattle, WA.
Funding: The authors gratefully acknowledge funding by NIH grants 1R03HDO75994 and K12 HD001259 for the conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors report no conflict of interest.
Contributor Information
Marianna Alperin, San Diego, California. Department of Reproductive Medicine, Division of Urogynecology and Pelvic Reconstructive Surgery, University of California, San Diego.
Mark Cook, Minneapolis, Minnesota. Department of Integrative Biology and Physiology, University of Minnesota.
Lori J. Tuttle, San Diego, California. Doctor of Physical Therapy Program, Exercise and Nutritional Sciences Physical Therapy, San Diego State University.
Mrs Mary C. Esparza, San Diego, California. San Diego, California. Department of Orthopaedic Surgery, University of California, San Diego.
Richard L. Lieber, San Diego, California and Chicago, Illinois. Departments of Orthopaedic Surgery and Bioengineering, University of California, San Diego. Rehabilitation Institute of Chicago.
References
- 1.DeLancey JOL. Pelvic Organ Prolapse: Clinical Management and Scientific Foundations. Clinical Obstetrics and Gynecology. 1993;36(4):895–896. [Google Scholar]
- 2.Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS. Cost of Pelvic Organ Prolapse Surgery in the United States. Obstetrics & Gynecology. 2001;98(4) doi: 10.1016/s0029-7844(01)01472-7. [DOI] [PubMed] [Google Scholar]
- 3.Wilson L, Brown JS, Shin GP, Luc K-O, Subak LL. Annual Direct Cost of Urinary Incontinence. Obstetrics & Gynecology. 2001;98(3) doi: 10.1016/s0029-7844(01)01464-8. [DOI] [PubMed] [Google Scholar]
- 4.Gregory WT, Nygaard I. Childbirth and Pelvic Floor Disorders. Clinical Obstetrics and Gynecology. 2004;47(2):394–403. doi: 10.1097/00003081-200406000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of Surgically Managed Pelvic Organ Prolapse and Urinary Incontinence. Obstetrics & Gynecology. 1997;89(4):501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 6.Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, et al. Prevalence of symptomatic pelvic floor disorders in US women. Jama. 2008;300(11):1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLancey J, Sørensen H, Lewicky-Gaupp C, Smith T. Comparison of the puborectal muscle on MRI in women with POP and levator ani defects with those with normal support and no defect. International urogynecology journal. 2012;23(1):73–77. doi: 10.1007/s00192-011-1527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLancey JOL, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, et al. Comparison of Levator Ani Muscle Defects and Function in Women With and Without Pelvic Organ Prolapse. Obstetrics & Gynecology. 2007;109(2, Part 1):295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 9.Hilde G, Staer-Jensen J, Siafarikas F, Engh ME, Braekken IH, Bo K. Impact of childbirth and mode of delivery on vaginal resting pressure and on pelvic floor muscle strength and endurance. American journal of obstetrics and gynecology. 2013;208(1):50. doi: 10.1016/j.ajog.2012.10.878. e1–7. [DOI] [PubMed] [Google Scholar]
- 10.Gameiro MO, Sousa VO, Gameiro LF, Muchailh RC, Padovani CR, Amaro JL. Clinics. 8. Vol. 66. Sao Paulo, Brazil: 2011. Comparison of pelvic floor muscle strength evaluations in nulliparous and primiparous women: a prospective study; pp. 1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson KA, Hsu Y, Chen L, Ashton-Miller JA, DeLancey JO. Magnetic resonance imaging-based three-dimensional model of anterior vaginal wall position at rest and maximal strain in women with and without prolapse. International urogynecology journal. 2010;21(9):1103–1109. doi: 10.1007/s00192-010-1161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle & nerve. 2000;23(11):1647–1666. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 13.Macaluso A, Nimmo MA, Foster JE, Cockburn M, McMillan NC, De Vito G. Contractile muscle volume and agonist-antagonist coactivation account for differences in torque between young and older women. Muscle & nerve. 2002;25(6):858–863. doi: 10.1002/mus.10113. [DOI] [PubMed] [Google Scholar]
- 14.Morse CI, Thom JM, Davis MG, Fox KR, Birch KM, Narici MV. Reduced plantarflexor specific torque in the elderly is associated with a lower activation capacity. European journal of applied physiology. 2004;92(1–2):219–226. doi: 10.1007/s00421-004-1056-y. [DOI] [PubMed] [Google Scholar]
- 15.Klein CS, Rice CL, Marsh GD. Normalized force, activation, and coactivation in the arm muscles of young and old men. Journal of applied physiology (Bethesda, Md : 1985) 2001;91(3):1341–1349. doi: 10.1152/jappl.2001.91.3.1341. [DOI] [PubMed] [Google Scholar]
- 16.Narici MV, Maganaris CN, Reeves ND, Capodaglio P. Effect of aging on human muscle architecture. Journal of applied physiology (Bethesda, Md : 1985) 2003;95(6):2229–2234. doi: 10.1152/japplphysiol.00433.2003. [DOI] [PubMed] [Google Scholar]
- 17.Morris VC, Murray MP, Delancey JO, Ashton-Miller JA. A comparison of the effect of age on levator ani and obturator internus muscle cross-sectional areas and volumes in nulliparous women. Neurourology and urodynamics. 2012;31(4):481–486. doi: 10.1002/nau.21208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuttle LJ, Nguyen OT, Cook MS, Alperin M, Shah SB, Ward SR, et al. Architectural design of the pelvic floor is consistent with muscle functional subspecialization. International urogynecology journal. 2014;25(2):205–212. doi: 10.1007/s00192-013-2189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latil M, Rocheteau P, Chatre L, Sanulli S, Memet S, Ricchetti M, et al. Skeletal muscle stem cells adopt a dormant cell state post mortem and retain regenerative capacity. Nature communications. 2012;3:903. doi: 10.1038/ncomms1890. [DOI] [PubMed] [Google Scholar]
- 20.Lieber RL. Biology and mechanics of skeletal muscle: what hand surgeons need to know when tensioning a tendon transfer. The Journal of hand surgery. 2008;33(9):1655–1656. doi: 10.1016/j.jhsa.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Lieber RL, Friden J. Clinical significance of skeletal muscle architecture. Clin Orthop Relat Res. 2001;(383):140–151. doi: 10.1097/00003086-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Lieber RL, Friden J. Implications of muscle design on surgical reconstruction of upper extremities. Clin Orthop Relat Res. 2004;(419):267–279. [PubMed] [Google Scholar]
- 23.Shek KL, Dietz HP. Intrapartum risk factors for levator trauma. BJOG : an international journal of obstetrics and gynaecology. 2010;117(12):1485–1492. doi: 10.1111/j.1471-0528.2010.02704.x. [DOI] [PubMed] [Google Scholar]
- 24.Albrich SB, Laterza RM, Skala C, Salvatore S, Koelbl H, Naumann G. Impact of mode of delivery on levator morphology: a prospective observational study with three-dimensional ultrasound early in the postpartum period. BJOG : an international journal of obstetrics and gynaecology. 2012;119(1):51–60. doi: 10.1111/j.1471-0528.2011.03152.x. [DOI] [PubMed] [Google Scholar]
- 25.Doumouchtsis SK, Chrysanthopoulou EL. Urogenital consequences in ageing women. Best practice & research Clinical obstetrics & gynaecology. 2013;27(5):699–714. doi: 10.1016/j.bpobgyn.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Schoenaker DA, Jackson CA, Rowlands JV, Mishra GD. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. International journal of epidemiology. 2014;43(5):1542–1562. doi: 10.1093/ije/dyu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. Journal of the neurological sciences. 1988;84(2–3):275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 28.Narici MV, Maganaris CN. Adaptability of elderly human muscles and tendons to increased loading. Journal of anatomy. 2006;208(4):433–443. doi: 10.1111/j.1469-7580.2006.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lieber RL, Fazeli BM, Botte MJ. Architecture of selected wrist flexor and extensor muscles. The Journal of hand surgery. 1990;15(2):244–250. doi: 10.1016/0363-5023(90)90103-x. [DOI] [PubMed] [Google Scholar]
- 30.Lieber RL, Yeh Y, Baskin RJ. Sarcomere length determination using laser diffraction Effect of beam and fiber diameter. Biophysical journal. 1984;45(5):1007–1016. doi: 10.1016/S0006-3495(84)84246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burkholder TJ, Lieber RL. Sarcomere length operating range of vertebrate muscles during movement. The Journal of experimental biology. 2001;204(Pt 9):1529–1536. doi: 10.1242/jeb.204.9.1529. [DOI] [PubMed] [Google Scholar]
- 32.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle & nerve. 2011;44(3):318–331. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purslow PP. Muscle fascia and force transmission. Journal of Bodywork and Movement Therapies. 2010;14(4):411–417. doi: 10.1016/j.jbmt.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Meyer GA, Lieber RL. Elucidation of extracellular matrix mechanics from muscle fibers and fiber bundles. Journal of biomechanics. 2011;44(4):771–773. doi: 10.1016/j.jbiomech.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86-A(9):1973–1982. doi: 10.2106/00004623-200409000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Edwards CA. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980:161–167. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- 37.Alperin M, Lawley DM, Esparza MC, Lieber RL. Pregnancy-induced adaptations in the intrinsic structure of rat pelvic floor muscles. American journal of obstetrics and gynecology. 2015 doi: 10.1016/j.ajog.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lieber RL. Statistical significance and statistical power in hypothesis testing. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1990;8(2):304–309. doi: 10.1002/jor.1100080221. [DOI] [PubMed] [Google Scholar]
- 39.Tuttle LJ, Ward SR, Lieber RL. Sample size considerations in human muscle architecture studies. Muscle & nerve. 2012;45(5):743–745. doi: 10.1002/mus.23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Boyle AL, O’Boyle JD, Calhoun B, Davis GD. Pelvic organ support in pregnancy and postpartum. International urogynecology journal and pelvic floor dysfunction. 2005;16(1):69–72. doi: 10.1007/s00192-004-1210-4. discussion. [DOI] [PubMed] [Google Scholar]
- 41.Reimers C, Staer-Jensen J, Siafarikas F, Saltyte-Benth J, Bo K, Ellstrom Engh M. Change in pelvic organ support during pregnancy and the first year postpartum: a longitudinal study. BJOG : an international journal of obstetrics and gynaecology. 2015 doi: 10.1111/1471-0528.13432. [DOI] [PubMed] [Google Scholar]
- 42.Elenskaia K, Thakar R, Sultan AH, Scheer I, Onwude J. Effect of childbirth on pelvic organ support and quality of life: a longitudinal cohort study. International urogynecology journal. 2013;24(6):927–937. doi: 10.1007/s00192-012-1932-7. [DOI] [PubMed] [Google Scholar]
- 43.Lieber RL, Ward SR. Cellular mechanisms of tissue fibrosis 4. Structural and functional consequences of skeletal muscle fibrosis. American journal of physiology Cell physiology. 2013;305(3):C241–C252. doi: 10.1152/ajpcell.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lieber RL, Runesson E, Einarsson F, Friden J. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle & nerve. 2003;28(4):464–471. doi: 10.1002/mus.10446. [DOI] [PubMed] [Google Scholar]
- 45.Järvinen TAH, Józsa L, Kannus P, Järvinen TLN, Järvinen M. Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles. Journal of Muscle Research and Cell Motility. 2002;23(3):245–254. doi: 10.1023/a:1020904518336. [DOI] [PubMed] [Google Scholar]
- 46.Sherburn M, Bird M, Carey M, Bo K, Galea MP. Incontinence improves in older women after intensive pelvic floor muscle training: an assessor-blinded randomized controlled trial. Neurourology and urodynamics. 2011;30(3):317–324. doi: 10.1002/nau.20968. [DOI] [PubMed] [Google Scholar]
- 47.Lewicky-Gaupp C, Brincat C, Yousuf A, Patel DA, Delancey JO, Fenner DE. Fecal incontinence in older women: are levator ani defects a factor? American journal of obstetrics and gynecology. 2010;202(5):491. doi: 10.1016/j.ajog.2010.01.020. e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kepenekci I, Keskinkilic B, Akinsu F, Cakir P, Elhan AH, Erkek AB, et al. Prevalence of pelvic floor disorders in the female population and the impact of age, mode of delivery, and parity. Diseases of the colon and rectum. 2011;54(1):85–94. doi: 10.1007/DCR.0b013e3181fd2356. [DOI] [PubMed] [Google Scholar]
- 49.Quiroz LH, Shobeiri SA, White D, Wild RA. Does age affect visualization of the levator ani in nulliparous women? International urogynecology journal. 2013;24(9):1507–1513. doi: 10.1007/s00192-013-2053-7. [DOI] [PubMed] [Google Scholar]
- 50.Janda tn, van der Helm FCT, de Blok SB. Measuring morphological parameters of the pelvic floor for finite element modelling purposes. Journal of Biomechanics. 2003;36(6):749–757. doi: 10.1016/s0021-9290(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 51.Felder A, Ward SR, Lieber RL. Sarcomere length measurement permits high resolution normalization of muscle fiber length in architectural studies. The Journal of experimental biology. 2005;208(Pt 17):3275–3279. doi: 10.1242/jeb.01763. [DOI] [PubMed] [Google Scholar]
- 52.Jundt K, Kiening M, Fischer P, Bergauer F, Rauch E, Janni W, et al. Is the histomorphological concept of the female pelvic floor and its changes due to age and vaginal delivery correct? Neurourology and urodynamics. 2005;24(1):44–50. doi: 10.1002/nau.20080. [DOI] [PubMed] [Google Scholar]
- 53.Dimpfl T, Jaeger C, Mueller-Felber W, Anthuber C, Hirsch A, Brandmaier R, et al. Myogenic changes of the levator ani muscle in premenopausal women: the impact of vaginal delivery and age. Neurourology and urodynamics. 1998;17(3):197–205. doi: 10.1002/(sici)1520-6777(1998)17:3<197::aid-nau4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 54.Vandervoort AA, McComas AJ. Contractile changes in opposing muscles of the human ankle joint with aging. Journal of applied physiology (Bethesda, Md : 1985) 1986;61(1):361–367. doi: 10.1152/jappl.1986.61.1.361. [DOI] [PubMed] [Google Scholar]